A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment
- PMID: 33620419
- PMCID: PMC7918756
- DOI: 10.1084/jem.20201637
A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment
Abstract
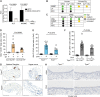
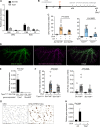
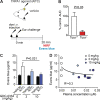
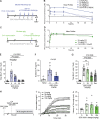
Despite the development of effective therapies, a substantial proportion of asthmatics continue to have uncontrolled symptoms, airflow limitation, and exacerbations. Transient receptor potential cation channel member A1 (TRPA1) agonists are elevated in human asthmatic airways, and in rodents, TRPA1 is involved in the induction of airway inflammation and hyperreactivity. Here, the discovery and early clinical development of GDC-0334, a highly potent, selective, and orally bioavailable TRPA1 antagonist, is described. GDC-0334 inhibited TRPA1 function on airway smooth muscle and sensory neurons, decreasing edema, dermal blood flow (DBF), cough, and allergic airway inflammation in several preclinical species. In a healthy volunteer Phase 1 study, treatment with GDC-0334 reduced TRPA1 agonist-induced DBF, pain, and itch, demonstrating GDC-0334 target engagement in humans. These data provide therapeutic rationale for evaluating TRPA1 inhibition as a clinical therapy for asthma.
© 2021 Genentech.
Figures





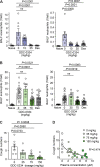
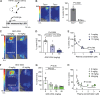

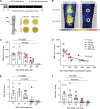
Comment in
-
TRPA1: An asthma target with a zing.J Exp Med. 2021 Apr 5;218(4):e20202507. doi: 10.1084/jem.20202507. J Exp Med. 2021. PMID: 33625497 Free PMC article.
Similar articles
-
Translational and pharmacokinetic-pharmacodynamic application for the clinical development of GDC-0334, a novel TRPA1 inhibitor.Clin Transl Sci. 2021 Sep;14(5):1945-1954. doi: 10.1111/cts.13049. Epub 2021 May 31. Clin Transl Sci. 2021. PMID: 34058071 Free PMC article. Clinical Trial.
-
A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma.Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9099-104. doi: 10.1073/pnas.0900591106. Epub 2009 May 19. Proc Natl Acad Sci U S A. 2009. PMID: 19458046 Free PMC article.
-
TRPA1 Channel as a Regulator of Neurogenic Inflammation and Pain: Structure, Function, Role in Pathophysiology, and Therapeutic Potential of Ligands.Biochemistry (Mosc). 2019 Feb;84(2):101-118. doi: 10.1134/S0006297919020020. Biochemistry (Mosc). 2019. PMID: 31216970 Review.
-
The novel TRPA1 antagonist BI01305834 inhibits ovalbumin-induced bronchoconstriction in guinea pigs.Respir Res. 2021 Feb 8;22(1):48. doi: 10.1186/s12931-021-01638-7. Respir Res. 2021. PMID: 33557843 Free PMC article.
-
Transient receptor potential ankyrin 1 (TRPA1) antagonists: a patent review (2015-2019).Expert Opin Ther Pat. 2020 Sep;30(9):643-657. doi: 10.1080/13543776.2020.1797679. Epub 2020 Aug 25. Expert Opin Ther Pat. 2020. PMID: 32686526 Review.
Cited by
-
A Comprehensive Review on the Regulatory Action of TRP Channels: A Potential Therapeutic Target for Nociceptive Pain.Neurosci Insights. 2023 Dec 24;18:26331055231220340. doi: 10.1177/26331055231220340. eCollection 2023. Neurosci Insights. 2023. PMID: 38146332 Free PMC article. Review.
-
Cytokines reprogram airway sensory neurons in asthma.bioRxiv [Preprint]. 2024 Sep 18:2023.01.26.525731. doi: 10.1101/2023.01.26.525731. bioRxiv. 2024. Update in: Cell Rep. 2024 Dec 24;43(12):115045. doi: 10.1016/j.celrep.2024.115045 PMID: 39345572 Free PMC article. Updated. Preprint.
-
Analysis of global gene expression using RNA-sequencing reveals novel mechanism of Yanghe Pingchuan decoction in the treatment of asthma.BMC Pulm Med. 2024 Mar 18;24(1):137. doi: 10.1186/s12890-024-02952-8. BMC Pulm Med. 2024. PMID: 38500104 Free PMC article.
-
Sensory TRP Channels in Three Dimensions.Annu Rev Biochem. 2022 Jun 21;91:629-649. doi: 10.1146/annurev-biochem-032620-105738. Epub 2022 Mar 14. Annu Rev Biochem. 2022. PMID: 35287474 Free PMC article. Review.
-
Advancing Lung Immunology Research: An Official American Thoracic Society Workshop Report.Am J Respir Cell Mol Biol. 2022 Jul;67(1):e1-18. doi: 10.1165/rcmb.2022-0167ST. Am J Respir Cell Mol Biol. 2022. PMID: 35776495 Free PMC article.
References
-
- Andrè, E., Campi B., Materazzi S., Trevisani M., Amadesi S., Massi D., Creminon C., Vaksman N., Nassini R., Civelli M., et al. . 2008. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118:2574–2582. - PMC - PubMed
-
- Baral, P., Umans B.D., Li L., Wallrapp A., Bist M., Kirschbaum T., Wei Y., Zhou Y., Kuchroo V.K., Burkett P.R., et al. . 2018. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24:417–426. 10.1038/nm.4501 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

