Analysis of Tandem Repeat Protein Folding Using Nearest-Neighbor Models
- PMID: 33606943
- PMCID: PMC8105288
- DOI: 10.1146/annurev-biophys-102220-083020
Analysis of Tandem Repeat Protein Folding Using Nearest-Neighbor Models
Abstract
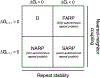
Cooperativity is a hallmark of protein folding, but the thermodynamic origins of cooperativity are difficult to quantify. Tandem repeat proteins provide a unique experimental system to quantify cooperativity due to their internal symmetry and their tolerance of deletion, extension, and in some cases fragmentation into single repeats. Analysis of repeat proteins of different lengths with nearest-neighbor Ising models provides values for repeat folding ([Formula: see text]) and inter-repeat coupling (ΔGi-1,i). In this article, we review the architecture of repeat proteins and classify them in terms of ΔGi and ΔGi-1,i; this classification scheme groups repeat proteins according to their degree of cooperativity. We then present various statistical thermodynamic models, based on the 1D-Ising model, for analysis of different classes of repeat proteins. We use these models to analyze data for highly and moderately cooperative and noncooperative repeat proteins and relate their fitted parameters to overall structural features.
Keywords: Ising model; cooperativity; protein folding; repeat protein; statistical thermodynamics.
Figures

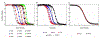

Similar articles
-
Stability Islands and the Folding Cooperativity of a Seven-Repeat Array from Topoisomerase V.J Am Chem Soc. 2023 Jun 14;145(23):12641-12650. doi: 10.1021/jacs.3c02193. Epub 2023 Jun 1. J Am Chem Soc. 2023. PMID: 37262333 Free PMC article.
-
Analysis of repeat-protein folding using nearest-neighbor statistical mechanical models.Methods Enzymol. 2009;455:95-125. doi: 10.1016/S0076-6879(08)04204-3. Methods Enzymol. 2009. PMID: 19289204 Free PMC article.
-
Repeat-protein folding: new insights into origins of cooperativity, stability, and topology.Arch Biochem Biophys. 2008 Jan 1;469(1):83-99. doi: 10.1016/j.abb.2007.08.034. Epub 2007 Sep 15. Arch Biochem Biophys. 2008. PMID: 17963718 Free PMC article. Review.
-
A collection of programs for one-dimensional Ising analysis of linear repeat proteins with point substitutions.Protein Sci. 2021 Jan;30(1):168-186. doi: 10.1002/pro.3977. Epub 2020 Nov 2. Protein Sci. 2021. PMID: 33058322 Free PMC article.
-
Folding cooperativity and allosteric function in the tandem-repeat protein class.Philos Trans R Soc Lond B Biol Sci. 2018 Jun 19;373(1749):20170188. doi: 10.1098/rstb.2017.0188. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29735741 Free PMC article. Review.
Cited by
-
Protein folding in vitro and in the cell: From a solitary journey to a team effort.Biophys Chem. 2022 Aug;287:106821. doi: 10.1016/j.bpc.2022.106821. Epub 2022 Apr 29. Biophys Chem. 2022. PMID: 35667131 Free PMC article. Review.
-
Evolution and folding of repeat proteins.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2204131119. doi: 10.1073/pnas.2204131119. Epub 2022 Jul 29. Proc Natl Acad Sci U S A. 2022. PMID: 35905321 Free PMC article.
-
Resolving the fine structure in the energy landscapes of repeat proteins.QRB Discov. 2022 Jun 10;3:e7. doi: 10.1017/qrd.2022.4. eCollection 2022. QRB Discov. 2022. PMID: 37529289 Free PMC article.
-
Stability Islands and the Folding Cooperativity of a Seven-Repeat Array from Topoisomerase V.J Am Chem Soc. 2023 Jun 14;145(23):12641-12650. doi: 10.1021/jacs.3c02193. Epub 2023 Jun 1. J Am Chem Soc. 2023. PMID: 37262333 Free PMC article.
References
-
- Arora P, Hammes GG, Oas TG. 2006. Folding Mechanism of a Multiple Independently-Folding Domain Protein: Double B Domain of Protein A†. Biochemistry. 45(40):12312–24 - PubMed
-
- Barrick D 2017. Biomolecular Thermodynamics: From Theory to Application. Boca Raton: CRC Press. 552 pp. 1 edition ed.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

