Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients
- PMID: 33594499
- PMCID: PMC8857941
- DOI: 10.1007/s00424-021-02525-2
Upregulation of transient receptor potential melastatin 4 (TRPM4) in ventricular fibroblasts from heart failure patients
Abstract
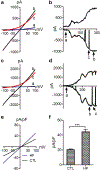
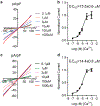
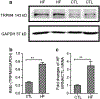
The transient receptor potential melastatin 4 (TRPM4) is a Ca2+-activated nonselective monovalent cation channel belonging to the TRP channel superfamily. TRPM4 is widely expressed in various tissues and most abundantly expressed in the heart. TRPM4 plays a critical role in cardiac conduction. Patients carrying a gain-of-function or loss-of-function mutation of TRPM4 display impaired cardiac conduction. Knockout or over-expression of TRPM4 in mice recapitulates conduction defects in patients. Moreover, recent studies have indicated that TRPM4 plays a role in hypertrophy and heart failure. Whereas the role of TRPM4 mediated by cardiac myocytes has been well investigated, little is known about TRPM4 and its role in cardiac fibroblasts. Here we show that in human left ventricular fibroblasts, TRPM4 exhibits typical Ca2+-activation characteristics, linear current-voltage (I-V) relation, and monovalent permeability. TRPM4 currents recorded in fibroblasts from heart failure patients (HF) are more than 2-fold bigger than those from control individuals (CTL). The enhanced functional TRPM4 in HF is not resulted from changed channel properties, as TRPM4 currents from both HF and CTL fibroblasts demonstrate similar sensitivity to intracellular calcium activation and extracellular 9-phenanthrol (9-phen) blockade. Consistent with enhanced TRPM4 activity, the protein level of TRPM4 is about 2-fold higher in HF than that of CTL hearts. Moreover, TRPM4 current in CTL fibroblasts is increased after 24 hours of TGFβ1 treatment, implying that TRPM4 in vivo may be upregulated by fibrogenesis promotor TGFβ1. The upregulated TRPM4 in HF fibroblasts suggests that TRPM4 may play a role in cardiac fibrogenesis under various pathological conditions.
Keywords: Calcium signaling; Heart failure; Human ventricular fibroblasts; TRP channels; TRPM4.
Figures



Similar articles
-
Electrophysiological Effects of the Transient Receptor Potential Melastatin 4 Channel Inhibitor (4-Chloro-2-(2-chlorophenoxy)acetamido) Benzoic Acid (CBA) in Canine Left Ventricular Cardiomyocytes.Int J Mol Sci. 2021 Aug 31;22(17):9499. doi: 10.3390/ijms22179499. Int J Mol Sci. 2021. PMID: 34502410 Free PMC article.
-
New role of TRPM4 channel in the cardiac excitation-contraction coupling in response to physiological and pathological hypertrophy in mouse.Prog Biophys Mol Biol. 2021 Jan;159:105-117. doi: 10.1016/j.pbiomolbio.2020.09.006. Epub 2020 Oct 5. Prog Biophys Mol Biol. 2021. PMID: 33031824
-
Shear stress activates monovalent cation channel transient receptor potential melastatin subfamily 4 in rat atrial myocytes via type 2 inositol 1,4,5-trisphosphate receptors and Ca(2+) release.J Physiol. 2016 Jun 1;594(11):2985-3004. doi: 10.1113/JP270887. Epub 2016 Feb 9. J Physiol. 2016. PMID: 26751048 Free PMC article.
-
TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology.Biochem Pharmacol. 2012 Oct 1;84(7):873-81. doi: 10.1016/j.bcp.2012.06.021. Epub 2012 Jun 27. Biochem Pharmacol. 2012. PMID: 22750058 Review.
-
TRPM4.Handb Exp Pharmacol. 2014;222:461-87. doi: 10.1007/978-3-642-54215-2_18. Handb Exp Pharmacol. 2014. PMID: 24756717 Review.
Cited by
-
Progress on role of ion channels of cardiac fibroblasts in fibrosis.Front Physiol. 2023 Mar 9;14:1138306. doi: 10.3389/fphys.2023.1138306. eCollection 2023. Front Physiol. 2023. PMID: 36969589 Free PMC article. Review.
-
Emerging role of transient receptor potential (TRP) ion channels in cardiac fibroblast pathophysiology.Front Physiol. 2022 Oct 6;13:968393. doi: 10.3389/fphys.2022.968393. eCollection 2022. Front Physiol. 2022. PMID: 36277180 Free PMC article. Review.
-
Modulation of the Cardiac Myocyte Action Potential by the Magnesium-Sensitive TRPM6 and TRPM7-like Current.Int J Mol Sci. 2021 Aug 14;22(16):8744. doi: 10.3390/ijms22168744. Int J Mol Sci. 2021. PMID: 34445449 Free PMC article.
-
The Dysfunction of Ca2+ Channels in Hereditary and Chronic Human Heart Diseases and Experimental Animal Models.Int J Mol Sci. 2023 Oct 27;24(21):15682. doi: 10.3390/ijms242115682. Int J Mol Sci. 2023. PMID: 37958665 Free PMC article. Review.
-
Calcium and Heart Failure: How Did We Get Here and Where Are We Going?Int J Mol Sci. 2021 Jul 9;22(14):7392. doi: 10.3390/ijms22147392. Int J Mol Sci. 2021. PMID: 34299010 Free PMC article. Review.
References
-
- Barbet G, Demion M, Moura IC, Serafini N, Leger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P (2008) The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol 9:1148–1156. 10.1038/ni.1648 - DOI - PMC - PubMed
-
- Barefield DY, McNamara JW, Lynch TL, Kuster DWD, Govindan S, Haar L, Wang Y, Taylor EN, Lorenz JN, Nieman ML, Zhu G, Luther PK, Varro A, Dobrev D, Ai X, Janssen PML, Kass DA, Jones WK, Gilbert RJ, Sadayappan S (2019) Ablation of the calpain-targeted site in cardiac myosin binding protein-C is cardioprotective during ischemia-reperfusion injury. J Mol Cell Cardiol 129:236–246. 10.1016/j.yjmcc.2019.03.006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL062426/HL/NHLBI NIH HHS/United States
- R01 HL147350/HL/NHLBI NIH HHS/United States
- P01-HL06426/HL/NHLBI NIH HHS/United States
- R01 HL078960/HL/NHLBI NIH HHS/United States
- R01-HL146744/HL/NHLBI NIH HHS/United States
- R01-HL143750/HL/NHLBI NIH HHS/United States
- 19TPA34890022/AHA/American Heart Association-American Stroke Association/United States
- R01 HL168728/HL/NHLBI NIH HHS/United States
- R01 AA024769/AA/NIAAA NIH HHS/United States
- R01-AA024769/NH/NIH HHS/United States
- R01 HL146744/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

