Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1
- PMID: 33589779
- PMCID: PMC8149296
- DOI: 10.1038/s41434-021-00219-z
Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1
Abstract
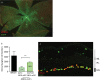
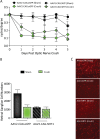
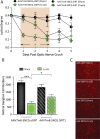
SIRT1 prevents retinal ganglion cell (RGC) loss in models of optic neuropathy following pharmacologic activation or genetic overexpression. The exact mechanism of loss is not known, prior evidence suggests this is through oxidative stress to either neighboring cells or RGC specifically. We investigated the neuroprotective potential of RGC-selective SIRT1 gene therapy in the optic nerve crush (ONC) model. We hypothesized that AAV-mediated overexpression of SIRT1 in RGCs reduces RGC loss, thereby preserving visual function. Cohorts of C57Bl/6J mice received intravitreal injection of experimental or control AAVs using either a ganglion cell promoter or a constitutive promoter and ONC was performed. Visual function was examined by optokinetic response (OKR) for 7 days following ONC. Retina and optic nerves were harvested to investigate RGC survival by immunolabeling. The AAV7m8-SNCG.SIRT1 vector showed 44% transduction efficiency for RGCs compared with 25% (P > 0.05) by AAV2-CAG.SIRT1, and AAV7m8-SNCG.SIRT1 drives expression selectively in RGCs in vivo. Animals modeling ONC demonstrated reduced visual acuity compared to controls. Intravitreal delivery of AAV7m8-SNCG.SIRT1 mediated significant preservation of the OKR and RGC survival compared to AAV7m8-SNCG.eGFP controls, an effect not seen with the AAV2 vector. RGC-selective expression of SIRT1 offers a targeted therapy for an animal model with significant ganglion cell loss. Over-expression of SIRT1 through AAV-mediated gene transduction suggests a RGC selective component of neuro-protection using the ONC model. This study expands our understanding of SIRT1 mediated neuroprotection in the context of compressive or traumatic optic neuropathy, making it a strong therapeutic candidate for testing in all optic neuropathies.
Conflict of interest statement
JB is a founder of Gensight Therapeutics and Limelight Bio and a scientific founder of Spark Therapeutics. JB receives a grant from Limelight Bio, is on the SAB for Akouos, and holds intellectual property relevant to this study. AGR, DSM, and KSS hold intellectual property relevant to this study. The other authors whose names are listed immediately below (RSK, TTD, KED, PA, VRMC) certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures

Similar articles
-
Cell-Specific Expression of Human SIRT1 by Gene Therapy Reduces Retinal Ganglion Cell Loss Induced by Elevated Intraocular Pressure.Neurotherapeutics. 2023 Apr;20(3):896-907. doi: 10.1007/s13311-023-01364-6. Epub 2023 Mar 20. Neurotherapeutics. 2023. PMID: 36941497 Free PMC article.
-
Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis.Biomolecules. 2022 Jun 14;12(6):830. doi: 10.3390/biom12060830. Biomolecules. 2022. PMID: 35740955 Free PMC article.
-
SIRT1 and NRF2 Gene Transfer Mediate Distinct Neuroprotective Effects Upon Retinal Ganglion Cell Survival and Function in Experimental Optic Neuritis.Invest Ophthalmol Vis Sci. 2018 Mar 1;59(3):1212-1220. doi: 10.1167/iovs.17-22972. Invest Ophthalmol Vis Sci. 2018. PMID: 29494741 Free PMC article.
-
Gene therapy and transplantation in the retinofugal pathway.Prog Brain Res. 2009;175:151-61. doi: 10.1016/S0079-6123(09)17510-6. Prog Brain Res. 2009. PMID: 19660654 Review.
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
Cited by
-
Long Non-Coding RNAs in Retinal Ganglion Cell Apoptosis.Cell Mol Neurobiol. 2023 Mar;43(2):561-574. doi: 10.1007/s10571-022-01210-x. Epub 2022 Feb 28. Cell Mol Neurobiol. 2023. PMID: 35226226 Review.
-
A Mini-Review on Gene Therapy in Glaucoma and Future Directions.Int J Mol Sci. 2024 Oct 14;25(20):11019. doi: 10.3390/ijms252011019. Int J Mol Sci. 2024. PMID: 39456800 Free PMC article. Review.
-
Comparison of SNCG and NEFH Promoter-Driven Expression of Human SIRT1 Expression in a Mouse Model of Glaucoma.Transl Vis Sci Technol. 2024 Aug 1;13(8):37. doi: 10.1167/tvst.13.8.37. Transl Vis Sci Technol. 2024. PMID: 39177995 Free PMC article.
-
AAV-NDI1 Therapy Provides Significant Benefit to Murine and Cellular Models of Glaucoma.Int J Mol Sci. 2024 Aug 15;25(16):8876. doi: 10.3390/ijms25168876. Int J Mol Sci. 2024. PMID: 39201561 Free PMC article.
-
Cell-Specific Expression of Human SIRT1 by Gene Therapy Reduces Retinal Ganglion Cell Loss Induced by Elevated Intraocular Pressure.Neurotherapeutics. 2023 Apr;20(3):896-907. doi: 10.1007/s13311-023-01364-6. Epub 2023 Mar 20. Neurotherapeutics. 2023. PMID: 36941497 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

