Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity
- PMID: 33589596
- PMCID: PMC7884686
- DOI: 10.1038/s41419-021-03458-5
Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity
Abstract
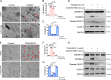
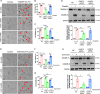
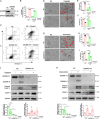
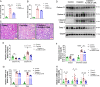
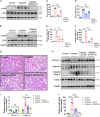
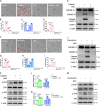
Chemotherapy drug-induced nephrotoxicity limits clinical applications for treating cancers. Pyroptosis, a newly discovered programmed cell death, was recently reported to be associated with kidney diseases. However, the role of pyroptosis in chemotherapeutic drug-induced nephrotoxicity has not been fully clarified. Herein, we demonstrate that the chemotherapeutic drug cisplatin or doxorubicin, induces the cleavage of gasdermin E (GSDME) in cultured human renal tubular epithelial cells, in a time- and concentration-dependent manner. Morphologically, cisplatin- or doxorubicin-treated renal tubular epithelial cells exhibit large bubbles emerging from the cell membrane. Furthermore, activation of caspase 3, not caspase 9, is associated with GSDME cleavage in cisplatin- or doxorubicin-treated renal tubular epithelial cells. Meanwhile, silencing GSDME alleviates cisplatin- or doxorubicin-induced HK-2 cell pyroptosis by increasing cell viability and decreasing LDH release. In addition, treatment with Ac-DMLD-CMK, a polypeptide targeting mouse caspase 3-Gsdme signaling, inhibits caspase 3 and Gsdme activation, alleviates the deterioration of kidney function, attenuates renal tubular epithelial cell injury, and reduces inflammatory cytokine secretion in vivo. Specifically, GSDME cleavage depends on ERK and JNK signaling. NAC, a reactive oxygen species (ROS) inhibitor, reduces GSDME cleavage through JNK signaling in human renal tubular epithelial cells. Thus, we speculate that renal tubular epithelial cell pyroptosis induced by chemotherapy drugs is mediated by ROS-JNK-caspase 3-GSDME signaling, implying that therapies targeting GSDME may prove efficacious in overcoming chemotherapeutic drug-induced nephrotoxicity.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Caspase-3-mediated GSDME activation contributes to cisplatin- and doxorubicin-induced secondary necrosis in mouse macrophages.Cell Prolif. 2019 Sep;52(5):e12663. doi: 10.1111/cpr.12663. Epub 2019 Jul 26. Cell Prolif. 2019. PMID: 31347748 Free PMC article.
-
Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation.Cell Death Dis. 2021 Feb 1;12(2):139. doi: 10.1038/s41419-021-03431-2. Cell Death Dis. 2021. PMID: 33542198 Free PMC article.
-
Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation.Apoptosis. 2019 Apr;24(3-4):312-325. doi: 10.1007/s10495-019-01515-1. Apoptosis. 2019. PMID: 30710195
-
Mini-Review: GSDME-Mediated Pyroptosis in Diabetic Nephropathy.Front Pharmacol. 2021 Nov 16;12:780790. doi: 10.3389/fphar.2021.780790. eCollection 2021. Front Pharmacol. 2021. PMID: 34867412 Free PMC article. Review.
-
The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review.Int J Biol Macromol. 2023 Jul 1;242(Pt 2):124832. doi: 10.1016/j.ijbiomac.2023.124832. Epub 2023 May 15. Int J Biol Macromol. 2023. PMID: 37196719 Review.
Cited by
-
Pyroptosis-related gene expression patterns and corresponding tumor microenvironment infiltration characterization in ovarian cancer.Comput Struct Biotechnol J. 2022 Sep 28;20:5440-5452. doi: 10.1016/j.csbj.2022.09.037. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36249562 Free PMC article.
-
Nephroprotective effects of diminazene on doxorubicin-induced acute kidney injury in rats.Toxicol Rep. 2023 Nov 10;11:460-468. doi: 10.1016/j.toxrep.2023.11.005. eCollection 2023 Dec. Toxicol Rep. 2023. PMID: 38053572 Free PMC article.
-
Osthole Induces Apoptosis and Caspase-3/GSDME-Dependent Pyroptosis via NQO1-Mediated ROS Generation in HeLa Cells.Oxid Med Cell Longev. 2022 Jun 8;2022:8585598. doi: 10.1155/2022/8585598. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35720178 Free PMC article.
-
GSDME-dependent pyroptosis signaling pathway in diabetic nephropathy.Cell Death Discov. 2023 May 11;9(1):156. doi: 10.1038/s41420-023-01452-8. Cell Death Discov. 2023. PMID: 37169767 Free PMC article.
-
Gasdermin E: A Prospective Target for Therapy of Diseases.Front Pharmacol. 2022 Apr 6;13:855828. doi: 10.3389/fphar.2022.855828. eCollection 2022. Front Pharmacol. 2022. PMID: 35462927 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

