Effectiveness of the Recombinant Zoster Vaccine in Adults Aged 50 and Older in the United States: A Claims-Based Cohort Study
- PMID: 33580245
- PMCID: PMC8442779
- DOI: 10.1093/cid/ciab121
Effectiveness of the Recombinant Zoster Vaccine in Adults Aged 50 and Older in the United States: A Claims-Based Cohort Study
Abstract
Background: The recombinant zoster vaccine had over 90% efficacy in preventing herpes zoster in clinical trials. However, its effectiveness outside of a clinical trial setting has not been investigated. This study aimed to assess the effectiveness of the recombinant zoster vaccine in general practice.
Methods: A de-identified administrative claims database, the OptumLabs Data Warehouse, was used to conduct this retrospective cohort study to assess the effectiveness of the recombinant zoster vaccine against herpes zoster in nonimmunocompromised, vaccine age-eligible individuals enrolled in the database for ≥365 days.
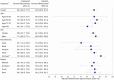
Results: A total of 4 769 819 adults were included in this study, with 173 745 (3.6%) adults receiving 2 valid doses of the recombinant zoster vaccine. The incidence rate of herpes zoster was 258.8 (95% confidence interval [CI], 230.0-289.4) cases per 100 000 person-years in vaccinated persons compared with 893.1 (95% CI, 886.2-900.0) in unvaccinated persons. Recombinant zoster vaccine effectiveness was 85.5% (95% CI, 83.5-87.3%) overall, with an effectiveness of 86.8% (95% CI, 84.6-88.7%) in individuals 50 to 79 years old compared with 80.3% (95% CI, 75.1-84.3%) in individuals aged 80 and older. In patients with a history of live zoster vaccine within 5 years of study inclusion, vaccine effectiveness was 84.8% (95% CI, 75.3-90.7%).
Conclusions: Recombinant zoster vaccine effectiveness against herpes zoster was high in a real-world setting. Given the low vaccine coverage and high effectiveness, a major public health effort is needed to identify and address barriers to vaccination and increase immunization rates.
Keywords: epidemiology; herpes zoster; infectious disease; real world evidence; recombinant zoster vaccine; shingrix vaccine; vaccine effectiveness.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
The Effectiveness of Recombinant Zoster Vaccine: Observations in the Wild.Clin Infect Dis. 2021 Sep 15;73(6):957-960. doi: 10.1093/cid/ciab130. Clin Infect Dis. 2021. PMID: 33580235 No abstract available.
Similar articles
-
Effectiveness of the Recombinant Zoster Vaccine for Herpes Zoster Ophthalmicus in the United States.Ophthalmology. 2021 Dec;128(12):1699-1707. doi: 10.1016/j.ophtha.2021.04.017. Epub 2021 Apr 20. Ophthalmology. 2021. PMID: 33892049 Free PMC article.
-
Effectiveness of the recombinant zoster vaccine among Kaiser Permanente Hawaii enrollees aged 50 and older: A retrospective cohort study.Vaccine. 2021 Jun 29;39(29):3974-3982. doi: 10.1016/j.vaccine.2021.05.056. Epub 2021 Jun 8. Vaccine. 2021. PMID: 34116874 Free PMC article.
-
Recombinant Zoster Vaccine (Shingrix): Real-World Effectiveness in the First 2 Years Post-Licensure.Clin Infect Dis. 2021 Sep 15;73(6):941-948. doi: 10.1093/cid/ciab125. Clin Infect Dis. 2021. PMID: 33580242
-
Vaccines for preventing herpes zoster in older adults.Cochrane Database Syst Rev. 2016 Mar 3;3(3):CD008858. doi: 10.1002/14651858.CD008858.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Nov 7;2019(11). doi: 10.1002/14651858.CD008858.pub4. PMID: 26937872 Free PMC article. Updated. Review.
-
A practitioner's guide to the recombinant zoster vaccine: review of national vaccination recommendations.Expert Rev Vaccines. 2021 Sep;20(9):1065-1075. doi: 10.1080/14760584.2021.1956906. Epub 2021 Sep 1. Expert Rev Vaccines. 2021. PMID: 34311643 Review.
Cited by
-
[Prevention by vaccination of adult patients with pulmonary diseases].Pneumologe (Berl). 2021;18(5):327-338. doi: 10.1007/s10405-021-00402-4. Epub 2021 Jul 12. Pneumologe (Berl). 2021. PMID: 34276271 Free PMC article. German.
-
Estimated Public Health Impact of the Recombinant Zoster Vaccine.Mayo Clin Proc Innov Qual Outcomes. 2021 May 26;5(3):596-604. doi: 10.1016/j.mayocpiqo.2021.03.006. eCollection 2021 Jun. Mayo Clin Proc Innov Qual Outcomes. 2021. PMID: 34195552 Free PMC article.
-
Vaccines for the Elderly and Vaccination Programs in Europe and the United States.Vaccines (Basel). 2024 May 22;12(6):566. doi: 10.3390/vaccines12060566. Vaccines (Basel). 2024. PMID: 38932295 Free PMC article. Review.
-
Risk factors for herpes zoster: should people with asthma or COPD be vaccinated?Respir Res. 2023 Jan 28;24(1):35. doi: 10.1186/s12931-022-02305-1. Respir Res. 2023. PMID: 36709298 Free PMC article. Review.
-
Effectiveness of the Recombinant Zoster Vaccine for Herpes Zoster Ophthalmicus in the United States.Ophthalmology. 2021 Dec;128(12):1699-1707. doi: 10.1016/j.ophtha.2021.04.017. Epub 2021 Apr 20. Ophthalmology. 2021. PMID: 33892049 Free PMC article.
References
-
- Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis 2019; 69:341–4. - PubMed
-
- Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: updated evidence from observational databases, 1991–2016. Clin Infect Dis 2020; 70:995–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

