Recombinant oncolytic adenovirus expressing a soluble PVR elicits long-term antitumor immune surveillance
- PMID: 33575467
- PMCID: PMC7851489
- DOI: 10.1016/j.omto.2020.11.001
Recombinant oncolytic adenovirus expressing a soluble PVR elicits long-term antitumor immune surveillance
Abstract
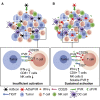
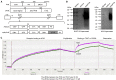
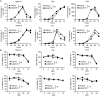
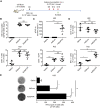
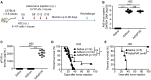
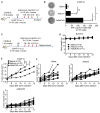
Oncolytic virotherapy (OVT) has been suggested to be effective. However, the suppressive effects of checkpoints and insufficient costimulatory signals limit OVT-induced antitumor immune responses. In this study, we constructed a replicative adenovirus, Ad5sPVR, that expresses the soluble extracellular domain of poliovirus receptor (sPVR). We showed that sPVR can bind to both T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) and CD226, and the binding affinity of sPVR to TIGIT is stronger than that of PVR to CD226. In the H22 hepatocellular carcinoma (HCC) ascites model, Ad5sPVR treatment increased the infiltration of CD8+ T cells and the release of interferon (IFN)-γ, exhibiting an antitumor effect with long-term tumor-specific immune surveillance. In line with this, Ad5sPVR also effectively improved antitumor outcomes in solid tumors. In conclusion, while Ad5sPVR plays a role in oncolysis and transforms cold tumors into hot tumors, sPVR expressed by Ad5sPVR can block the PVR/TIGIT checkpoint and activate CD226, thereby greatly improving the efficacy of OVT. This study provides a new way to develop potential oncolytic viral drugs.
Keywords: CD226; PVR/CD155; T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain; TIGIT; cancer therapy; immune checkpoints; oncolytic virus.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Recombinant adenovirus expressing the fusion protein PD1PVR improves CD8+ T cell-mediated antitumor efficacy with long-term tumor-specific immune surveillance in hepatocellular carcinoma.Cell Oncol (Dordr). 2021 Dec;44(6):1243-1255. doi: 10.1007/s13402-021-00633-w. Epub 2021 Sep 7. Cell Oncol (Dordr). 2021. PMID: 34491549
-
An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade.J Immunother Cancer. 2021 Dec;9(12):e002843. doi: 10.1136/jitc-2021-002843. J Immunother Cancer. 2021. PMID: 34949694 Free PMC article.
-
Perturbed CD8+ T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals.Sci Rep. 2017 Jan 13;7:40354. doi: 10.1038/srep40354. Sci Rep. 2017. PMID: 28084312 Free PMC article.
-
TIGIT-CD226-PVR axis: advancing immune checkpoint blockade for cancer immunotherapy.J Immunother Cancer. 2022 Apr;10(4):e004711. doi: 10.1136/jitc-2022-004711. J Immunother Cancer. 2022. PMID: 35379739 Free PMC article. Review.
-
TIGIT/CD226 Axis Regulates Anti-Tumor Immunity.Pharmaceuticals (Basel). 2021 Feb 28;14(3):200. doi: 10.3390/ph14030200. Pharmaceuticals (Basel). 2021. PMID: 33670993 Free PMC article. Review.
Cited by
-
Engineering strategies to enhance oncolytic viruses in cancer immunotherapy.Signal Transduct Target Ther. 2022 Apr 6;7(1):117. doi: 10.1038/s41392-022-00951-x. Signal Transduct Target Ther. 2022. PMID: 35387984 Free PMC article. Review.
-
Role of CD155/TIGIT in Digestive Cancers: Promising Cancer Target for Immunotherapy.Front Oncol. 2022 Mar 30;12:844260. doi: 10.3389/fonc.2022.844260. eCollection 2022. Front Oncol. 2022. PMID: 35433470 Free PMC article. Review.
-
Formin protein FMNL1 is a biomarker for tumor-infiltrating immune cells and associated with well immunotherapeutic response.J Cancer. 2023 Sep 11;14(16):2978-2989. doi: 10.7150/jca.86965. eCollection 2023. J Cancer. 2023. PMID: 37859818 Free PMC article.
-
The IgV domain of the poliovirus receptor alone is immunosuppressive and binds to its receptors with comparable affinity.Sci Rep. 2023 Mar 21;13(1):4609. doi: 10.1038/s41598-023-30999-w. Sci Rep. 2023. PMID: 36944702 Free PMC article.
-
CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy.Oncoimmunology. 2022 Aug 31;11(1):2118210. doi: 10.1080/2162402X.2022.2118210. eCollection 2022. Oncoimmunology. 2022. PMID: 36092638 Free PMC article.
References
-
- Keller B.A., Bell J.C. Oncolytic viruses—immunotherapeutics on the rise. J. Mol. Med. (Berl.) 2016;94:979–991. - PubMed
-
- Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. - PubMed
-
- Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. - PubMed
-
- Niemann J., Kuhnel F. Oncolytic viruses: adenoviruses. Virus Genes. 2017;53:700–706. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

