Dissociation of the respiratory syncytial virus F protein-specific human IgG, IgA and IgM response
- PMID: 33574352
- PMCID: PMC7878790
- DOI: 10.1038/s41598-021-82893-y
Dissociation of the respiratory syncytial virus F protein-specific human IgG, IgA and IgM response
Abstract
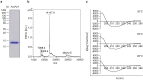
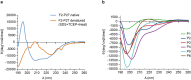
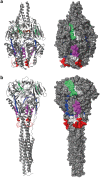
Human respiratory syncytial virus (RSV) is one of the most important causes of severe respiratory tract infections in early childhood. The only prophylactic protection is the neutralizing antibody, palivizumab, which targets a conformational epitope of the RSV fusion (F) protein. The F protein is generated as a F0 precursor containing two furin cleavage sites allowing excision of the P27 fragment and then gives rise to a fusion-competent version consisting of the N-terminal F2 subunit and the a C-terminal F1 subunits linked by two disulphide bonds. To investigate natural human F-specific antibody responses, F2 conferring the species-specificity of RSV, was expressed in Escherichia coli. Furthermore, the F0 protein, comprising both subunits F2 and F1, was expressed as palivizumab-reactive glycoprotein in baculovirus-infected insect cells. Six overlapping F2-derived peptides lacking secondary structure were synthesized. The analysis of IgG, IgA and IgM responses of adult subjects to native versions and denatured forms of F2 and F0 and to unfolded F2-derived peptides revealed that mainly non-conformational F epitopes, some of which represented cryptic epitopes which are not exposed on the proteins were recognized. Furthermore, we found a dissociation of IgG, IgA and IgM antibody responses to F epitopes with F2 being a major target for the F-specific IgM response. The scattered and dissociated immune response to F may explain why the natural RSV-specific antibody response is only partially protective underlining the need for vaccines focusing human antibody responses towards neutralizing RSV epitopes.
Conflict of interest statement
Rudolf Valenta has received research grants from Viravaxx, Vienna, Austria and serves as a consultant for this company. The other authors have no conflicts of interest to report.
Figures





Similar articles
-
Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: Structure, antigenic profile, immunogenicity, and protection.Vaccine. 2019 Sep 24;37(41):6112-6124. doi: 10.1016/j.vaccine.2019.07.089. Epub 2019 Aug 12. Vaccine. 2019. PMID: 31416644
-
Potent Human Single-Domain Antibodies Specific for a Novel Prefusion Epitope of Respiratory Syncytial Virus F Glycoprotein.J Virol. 2021 Aug 25;95(18):e0048521. doi: 10.1128/JVI.00485-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34160257 Free PMC article.
-
Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector.J Virol. 2016 Oct 14;90(21):10022-10038. doi: 10.1128/JVI.01196-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581977 Free PMC article.
-
Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches.Eur J Clin Microbiol Infect Dis. 2018 Oct;37(10):1817-1827. doi: 10.1007/s10096-018-3289-4. Epub 2018 Jun 6. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29876771 Review.
-
Clinical Potential of Prefusion RSV F-specific Antibodies.Trends Microbiol. 2018 Mar;26(3):209-219. doi: 10.1016/j.tim.2017.09.009. Epub 2017 Oct 17. Trends Microbiol. 2018. PMID: 29054341 Review.
Cited by
-
RSV Prevention in All Infants: Which Is the Most Preferable Strategy?Front Immunol. 2022 Apr 28;13:880368. doi: 10.3389/fimmu.2022.880368. eCollection 2022. Front Immunol. 2022. PMID: 35572550 Free PMC article. Review.
-
Development and comparison of immunologic assays to detect primary RSV infections in infants.Front Immunol. 2024 Jan 12;14:1332772. doi: 10.3389/fimmu.2023.1332772. eCollection 2023. Front Immunol. 2024. PMID: 38283339 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

