p53 dynamics vary between tissues and are linked with radiation sensitivity
- PMID: 33563973
- PMCID: PMC7873198
- DOI: 10.1038/s41467-021-21145-z
p53 dynamics vary between tissues and are linked with radiation sensitivity
Abstract
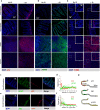
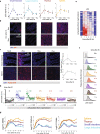
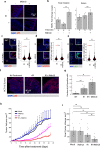
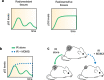
Radiation sensitivity varies greatly between tissues. The transcription factor p53 mediates the response to radiation; however, the abundance of p53 protein does not correlate well with the extent of radiosensitivity across tissues. Given recent studies showing that the temporal dynamics of p53 influence the fate of cultured cells in response to irradiation, we set out to determine the dynamic behavior of p53 and its impact on radiation sensitivity in vivo. We find that radiosensitive tissues show prolonged p53 signaling after radiation, while more resistant tissues show transient p53 activation. Sustaining p53 using a small molecule (NMI801) that inhibits Mdm2, a negative regulator of p53, reduced viability in cell culture and suppressed tumor growth. Our work proposes a mechanism for the control of radiation sensitivity and suggests tools to alter the dynamics of p53 to enhance tumor clearance. Similar approaches can be used to enhance killing of cancer cells or reduce toxicity in normal tissues following genotoxic therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Small Molecule Inhibition of MDM2-p53 Interaction Augments Radiation Response in Human Tumors.Mol Cancer Ther. 2015 Sep;14(9):1994-2003. doi: 10.1158/1535-7163.MCT-14-1056-T. Epub 2015 Jul 10. Mol Cancer Ther. 2015. PMID: 26162687
-
MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models.Cancer Res. 2013 Apr 15;73(8):2587-97. doi: 10.1158/0008-5472.CAN-12-2807. Epub 2013 Feb 11. Cancer Res. 2013. PMID: 23400593
-
MDM2 Inhibition Sensitizes Prostate Cancer Cells to Androgen Ablation and Radiotherapy in a p53-Dependent Manner.Neoplasia. 2016 Apr;18(4):213-22. doi: 10.1016/j.neo.2016.01.006. Neoplasia. 2016. PMID: 27108384 Free PMC article.
-
Nutlins and ionizing radiation in cancer therapy.Curr Pharm Des. 2010;16(12):1427-42. doi: 10.2174/138161210791033932. Curr Pharm Des. 2010. PMID: 20166982 Review.
-
Targeting the p53-MDM2 interaction to treat cancer.Br J Cancer. 2004 Oct 18;91(8):1415-9. doi: 10.1038/sj.bjc.6602164. Br J Cancer. 2004. PMID: 15452548 Free PMC article. Review.
Cited by
-
At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity.Int J Mol Sci. 2021 Oct 8;22(19):10883. doi: 10.3390/ijms221910883. Int J Mol Sci. 2021. PMID: 34639222 Free PMC article. Review.
-
Transcriptional pausing induced by ionizing radiation enables the acquisition of radioresistance in nasopharyngeal carcinoma.J Mol Cell Biol. 2024 Jan 5;15(7):mjad044. doi: 10.1093/jmcb/mjad044. J Mol Cell Biol. 2024. PMID: 37407287 Free PMC article.
-
Impact of Proton Irradiation Depending on Breast Cancer Subtype in Patient-Derived Cell Lines.Int J Mol Sci. 2024 Sep 29;25(19):10494. doi: 10.3390/ijms251910494. Int J Mol Sci. 2024. PMID: 39408826 Free PMC article.
-
Enhanced Effects of Chronic Restraint-Induced Psychological Stress on Total Body Fe-Irradiation-Induced Hematopoietic Toxicity in Trp53-Heterozygous Mice.Life (Basel). 2022 Apr 10;12(4):565. doi: 10.3390/life12040565. Life (Basel). 2022. PMID: 35455056 Free PMC article.
-
Small Molecules and Immunotherapy Agents for Enhancing Radiotherapy in Glioblastoma.Biomedicines. 2022 Jul 21;10(7):1763. doi: 10.3390/biomedicines10071763. Biomedicines. 2022. PMID: 35885067 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

