IL-36γ-armed oncolytic virus exerts superior efficacy through induction of potent adaptive antitumor immunity
- PMID: 33538860
- PMCID: PMC8360872
- DOI: 10.1007/s00262-021-02860-4
IL-36γ-armed oncolytic virus exerts superior efficacy through induction of potent adaptive antitumor immunity
Abstract
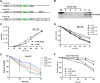
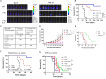
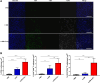
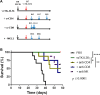
In this study, we aimed to apply the cytokine IL-36γ to cancer immunotherapy by constructing new oncolytic vaccinia viruses (OV) expressing interleukin-36γ (IL-36γ-OVs), leveraging unique synergism between OV and IL-36γ's ability to promote antitumor adaptive immunity and modulate tumor microenvironment (TME). IL-36γ-OV had dramatic therapeutic efficacies in multiple murine tumor models, frequently leading to complete cancer eradication in large fractions of mice. Mechanistically, IL-36-γ-armed OV induced infiltration of lymphocytes and dendritic cells, decreased myeloid-derived suppressor cells and M2-like tumor-associated macrophages, and T cell differentiation into effector cells. Further study showed that IL-36γ-OV increased the number of tumor antigen-specific CD4+ and CD8+ T cells and the therapeutic efficacy depended on both CD8+ and CD4+ T cells. These results demonstrate that these IL36γ-armed OVs exert potent therapeutic efficacy mainly though antitumor immunity and they may hold great potential to advance treatment in human cancer patients.
Keywords: CD4+ T cells; CD8+ T cells; Effector memory T cells; IL-36γ; Immunotherapy; Oncolytic virus.
© 2021. The Author(s).
Conflict of interest statement
A patent partly based on this work has been filed by DLB, BL and ZSG. All other authors declare no conflicts of interest.
Figures


Similar articles
-
Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation.Theranostics. 2021 May 3;11(14):6668-6681. doi: 10.7150/thno.56494. eCollection 2021. Theranostics. 2021. PMID: 34093846 Free PMC article.
-
Combining IL-10 and Oncolytic Adenovirus Demonstrates Enhanced Antitumor Efficacy Through CD8+ T Cells.Front Immunol. 2021 Feb 26;12:615089. doi: 10.3389/fimmu.2021.615089. eCollection 2021. Front Immunol. 2021. PMID: 33717103 Free PMC article.
-
Superagonist IL-15-Armed Oncolytic Virus Elicits Potent Antitumor Immunity and Therapy That Are Enhanced with PD-1 Blockade.Mol Ther. 2018 Oct 3;26(10):2476-2486. doi: 10.1016/j.ymthe.2018.07.013. Epub 2018 Jul 17. Mol Ther. 2018. PMID: 30064894 Free PMC article.
-
Oncolytic virus therapy: A new era of cancer treatment at dawn.Cancer Sci. 2016 Oct;107(10):1373-1379. doi: 10.1111/cas.13027. Epub 2016 Sep 9. Cancer Sci. 2016. PMID: 27486853 Free PMC article. Review.
-
Oncolytic viruses-immunotherapeutics on the rise.J Mol Med (Berl). 2016 Sep;94(9):979-91. doi: 10.1007/s00109-016-1453-9. Epub 2016 Aug 4. J Mol Med (Berl). 2016. PMID: 27492706 Review.
Cited by
-
Improving the therapeutic efficacy of oncolytic viruses for cancer: targeting macrophages.J Transl Med. 2023 Nov 22;21(1):842. doi: 10.1186/s12967-023-04709-z. J Transl Med. 2023. PMID: 37993941 Free PMC article. Review.
-
Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective.Viruses. 2023 Jul 28;15(8):1645. doi: 10.3390/v15081645. Viruses. 2023. PMID: 37631987 Free PMC article. Review.
-
In vitro Immunostimulant Activity of the Polyphenolic Extract from the Arctic Brown Algae Fucus vesiculosus.Plant Foods Hum Nutr. 2024 Jun;79(2):511-517. doi: 10.1007/s11130-024-01174-x. Epub 2024 Apr 13. Plant Foods Hum Nutr. 2024. PMID: 38613704
-
Recent progress in combination therapy of oncolytic vaccinia virus.Front Immunol. 2024 Mar 13;15:1272351. doi: 10.3389/fimmu.2024.1272351. eCollection 2024. Front Immunol. 2024. PMID: 38558795 Free PMC article. Review.
-
Autonomous IL-36R signaling in neutrophils activates potent antitumor effector functions.J Clin Invest. 2023 Jun 15;133(12):e162088. doi: 10.1172/JCI162088. J Clin Invest. 2023. PMID: 37317970 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

