Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19
- PMID: 33522572
- PMCID: PMC7953947
- DOI: 10.1093/humrep/deab026
Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19
Abstract
Study question: How is the semen quality of sexually active men following recovery from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection?
Summary answer: Twenty-five percent of the men with recent SARS-Cov-2 infections and proven healing were oligo-crypto-azoospermic, despite the absence of virus RNA in semen.
What is known already: The presence of SARS-CoV-2 in human semen and its role in virus contagion and semen quality after recovery from coronavirus disease 2019 (COVID-19) is still unclear. So far, studies evaluating semen quality and the occurrence of SARS-CoV-2 in semen of infected or proven recovered men are scarce and included a limited number of participants.
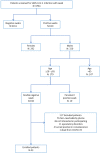
Study design, size, duration: A prospective cross-sectional study on 43 sexually active men who were known to have recovered from SARS-CoV2 was performed. Four biological fluid samples, namely saliva, pre-ejaculation urine, semen, and post-ejaculation urine, were tested for the SARS-CoV-2 genome. Female partners were retested if any specimen was found to be SARS-CoV-2 positive. Routine semen analysis and quantification of semen leukocytes and interleukin-8 (IL-8) levels were performed.
Participants/materials, setting, methods: Questionnaires including International Index of Erectile Function and Male Sexual Health Questionnaire Short Form were administered to all subjects. The occurrence of virus RNA was evaluated in all the biological fluids collected by RT-PCR. Semen parameters were evaluated according to the World Health Organization manual edition V. Semen IL-8 levels were evaluated by a two-step ELISA method.
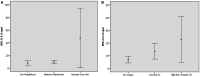
Main results and the role of chance: After recovery from COVID-19, 25% of the men studied were oligo-crypto-azoospermic. Of the 11 men with semen impairment, 8 were azoospermic and 3 were oligospermic. A total of 33 patients (76.7%) showed pathological levels of IL-8 in semen. Oligo-crypto-azoospermia was significantly related to COVID-19 severity (P < 0.001). Three patients (7%) tested positive for at least one sample (one saliva; one pre-ejaculation urine; one semen and one post-ejaculation urine), so the next day new nasopharyngeal swabs were collected. The results from these three patients and their partners were all negative for SARS-CoV-2.
Limitations, reasons for caution: Although crypto-azoospermia was found in a high percentage of men who had recovered from COVID-19, clearly exceeding the percentage found in the general population, the previous semen quality of these men was unknown nor is it known whether a recovery of testicular function was occurring. The low number of enrolled patients may limit the statistical power of study.
Wider implications of the findings: SARS-CoV-2 can be detected in saliva, urine, and semen in a small percentage of men who recovered from COVID-19. One-quarter of men who recovered from COVID-19 demonstrated oligo-crypto-azoospermia indicating that an assessment of semen quality should be recommended for men of reproductive age who are affected by COVID-19.
Study funding/competing interest(s): None.
Trial registration number: N/A.
Keywords: COVID-19; SARS-CoV-2; coronavirus disease 2019; fertility; oligo-crypto-azoospermia; semen; severe acute respiratory syndrome coronavirus 2; sexual transmission.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
COVID-19: semen impairment may not be related to the virus.Hum Reprod. 2021 Jun 18;36(7):2063-2064. doi: 10.1093/humrep/deab082. Hum Reprod. 2021. PMID: 33793791 Free PMC article. No abstract available.
-
Male Infertility.J Urol. 2022 Feb;207(2):449-451. doi: 10.1097/JU.0000000000002320. Epub 2021 Nov 17. J Urol. 2022. PMID: 34784732 No abstract available.
Similar articles
-
Risk of contamination of semen, vaginal secretions, follicular fluid and ovarian medulla with SARS-CoV-2 in patients undergoing ART.Hum Reprod. 2022 Jan 28;37(2):235-241. doi: 10.1093/humrep/deab255. Hum Reprod. 2022. PMID: 34741508 Free PMC article.
-
Semen quality impairment is associated with sexual dysfunction according to its severity.Hum Reprod. 2016 Dec;31(12):2668-2680. doi: 10.1093/humrep/dew246. Epub 2016 Oct 12. Hum Reprod. 2016. PMID: 27733531
-
No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation.Andrology. 2021 Jan;9(1):99-106. doi: 10.1111/andr.12939. Epub 2020 Nov 23. Andrology. 2021. PMID: 33150723
-
Comparative analysis of viral infection outcomes in human seminal fluid from prior viral epidemics and Sars-CoV-2 may offer trends for viral sexual transmissibility and long-term reproductive health implications.Reprod Health. 2021 Jun 10;18(1):123. doi: 10.1186/s12978-021-01172-1. Reprod Health. 2021. PMID: 34112171 Free PMC article. Review.
-
Twenty-First Century Viral Pandemics: A Literature Review of Sexual Transmission and Fertility Implications in Men.Sex Med Rev. 2020 Oct;8(4):518-530. doi: 10.1016/j.sxmr.2020.06.003. Epub 2020 Jul 24. Sex Med Rev. 2020. PMID: 32713674 Free PMC article. Review.
Cited by
-
Investigating the mode of transmission of COVID-19 through genital secretions, semen, the birth canal, and lactation: A systematic review.J Educ Health Promot. 2024 Jul 29;13:263. doi: 10.4103/jehp.jehp_387_23. eCollection 2024. J Educ Health Promot. 2024. PMID: 39309991 Free PMC article.
-
A Systemic Review and Meta-analysis of the Effect of SARS-CoV-2 Infection on Sperm Parameters.Research (Wash D C). 2022 Jul 13;2022:9835731. doi: 10.34133/2022/9835731. eCollection 2022. Research (Wash D C). 2022. PMID: 39301505 Free PMC article. Review.
-
SARS-CoV-2 impairs male fertility by targeting semen quality and testosterone level: A systematic review and meta-analysis.PLoS One. 2024 Sep 9;19(9):e0307396. doi: 10.1371/journal.pone.0307396. eCollection 2024. PLoS One. 2024. PMID: 39250513 Free PMC article.
-
Expression of SARS-CoV-2 entry molecules ACE2, NRP1, TMPRSS2, and FURIN in the reproductive tissues of male macaques.Histochem Cell Biol. 2024 Dec;162(6):465-475. doi: 10.1007/s00418-024-02314-9. Epub 2024 Aug 17. Histochem Cell Biol. 2024. PMID: 39153130
-
SARS-CoV-2 Vaccination Improves Semen Quality in Men Recovered From COVID-19: A Retrospective Cohort Study.Am J Mens Health. 2024 Jul-Aug;18(4):15579883241264120. doi: 10.1177/15579883241264120. Am J Mens Health. 2024. PMID: 39054777 Free PMC article.
References
-
- Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE.. History of febrile illness and variation in semen quality. Hum Reprod 2003;18:2089–2092. - PubMed
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU); https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594... (1 October 2020, date last accessed).
-
- Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C.. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol 2013;100:20–29. - PubMed
-
- Grande G, Milardi D, Baroni S, Luca G, Pontecorvi A.. Identification of seminal markers of male accessory gland inflammation: from molecules to proteome. Am J Reprod Immunol 2018;80:e12992. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

