Tumor-associated myeloid cells: diversity and therapeutic targeting
- PMID: 33473192
- PMCID: PMC8027665
- DOI: 10.1038/s41423-020-00613-4
Tumor-associated myeloid cells: diversity and therapeutic targeting
Abstract
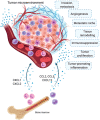
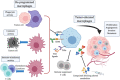
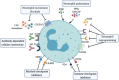
Myeloid cells in tumor tissues constitute a dynamic immune population characterized by a non-uniform phenotype and diverse functional activities. Both tumor-associated macrophages (TAMs), which are more abundantly represented, and tumor-associated neutrophils (TANs) are known to sustain tumor cell growth and invasion, support neoangiogenesis and suppress anticancer adaptive immune responses. In recent decades, several therapeutic approaches have been implemented in preclinical cancer models to neutralize the tumor-promoting roles of both TAMs and TANs. Some of the most successful strategies have now reached the clinic and are being investigated in clinical trials. In this review, we provide an overview of the recent literature on the ever-growing complexity of the biology of TAMs and TANs and the development of the most promising approaches to target these populations therapeutically in cancer patients.
Keywords: macrophage targeting; tumor microenvironment; tumor-associated macrophages.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Diamonds in the Rough: Harnessing Tumor-Associated Myeloid Cells for Cancer Therapy.Front Immunol. 2018 Oct 8;9:2250. doi: 10.3389/fimmu.2018.02250. eCollection 2018. Front Immunol. 2018. PMID: 30349530 Free PMC article. Review.
-
Research trends in pharmacological modulation of tumor-associated macrophages.Clin Transl Med. 2021 Jan;11(1):e288. doi: 10.1002/ctm2.288. Clin Transl Med. 2021. PMID: 33463063 Free PMC article. Review.
-
Tumor-associated macrophages: A promising target for a cancer immunotherapeutic strategy.Pharmacol Res. 2020 Nov;161:105111. doi: 10.1016/j.phrs.2020.105111. Epub 2020 Oct 13. Pharmacol Res. 2020. PMID: 33065284 Review.
-
Innate immune cells in the tumor microenvironment.Cancer Cell. 2021 Jun 14;39(6):725-729. doi: 10.1016/j.ccell.2021.05.016. Cancer Cell. 2021. PMID: 34129817
-
Targeting tumor-associated macrophages as an antitumor strategy.Biochem Pharmacol. 2021 Jan;183:114354. doi: 10.1016/j.bcp.2020.114354. Epub 2020 Dec 3. Biochem Pharmacol. 2021. PMID: 33279498 Review.
Cited by
-
Targeting tumour-reprogrammed myeloid cells: the new battleground in cancer immunotherapy.Semin Immunopathol. 2023 Mar;45(2):163-186. doi: 10.1007/s00281-022-00965-1. Epub 2022 Sep 26. Semin Immunopathol. 2023. PMID: 36161514 Free PMC article. Review.
-
Co-enrichment of CD8-positive T cells and macrophages is associated with clinical benefit of tislelizumab in solid tumors.Biomark Res. 2023 Mar 7;11(1):25. doi: 10.1186/s40364-023-00465-w. Biomark Res. 2023. PMID: 36879284 Free PMC article.
-
The complex role of tumor-infiltrating macrophages.Nat Immunol. 2022 Aug;23(8):1148-1156. doi: 10.1038/s41590-022-01267-2. Epub 2022 Jul 25. Nat Immunol. 2022. PMID: 35879449 Free PMC article. Review.
-
Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy.Cancers (Basel). 2022 Jan 28;14(3):680. doi: 10.3390/cancers14030680. Cancers (Basel). 2022. PMID: 35158948 Free PMC article. Review.
-
A topology perspective on macrophages in melanoma metastasis.Cell Rep Med. 2022 May 17;3(5):100643. doi: 10.1016/j.xcrm.2022.100643. Cell Rep Med. 2022. PMID: 35584636 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

