IgA and FcαRI: Versatile Players in Homeostasis, Infection, and Autoimmunity
- PMID: 33447585
- PMCID: PMC7801909
- DOI: 10.2147/ITT.S266242
IgA and FcαRI: Versatile Players in Homeostasis, Infection, and Autoimmunity
Abstract
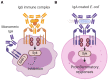
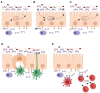
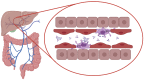
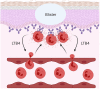
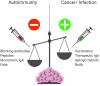
Mucosal surfaces constitute the frontiers of the body and are the biggest barriers of our body for the outside world. Immunoglobulin A (IgA) is the most abundant antibody class present at these sites. It passively contributes to mucosal homeostasis via immune exclusion maintaining a tight balance between tolerating commensals and providing protection against pathogens. Once pathogens have succeeded in invading the epithelial barriers, IgA has an active role in host-pathogen defense by activating myeloid cells through divers receptors, including its Fc receptor, FcαRI (CD89). To evade elimination, several pathogens secrete proteins that interfere with either IgA neutralization or FcαRI-mediated immune responses, emphasizing the importance of IgA-FcαRI interactions in preventing infection. Depending on the IgA form, either anti- or pro-inflammatory responses can be induced. Moreover, the presence of excessive IgA immune complexes can result in continuous FcαRI-mediated activation of myeloid cells, potentially leading to severe tissue damage. On the one hand, enhancing pathogen-specific mucosal and systemic IgA by vaccination may increase protective immunity against infectious diseases. On the other hand, interfering with the IgA-FcαRI axis by monovalent targeting or blocking FcαRI may resolve IgA-induced inflammation and tissue damage. This review describes the multifaceted role of FcαRI as immune regulator between anti- and pro-inflammatory responses of IgA, and addresses potential novel therapeutic strategies that target FcαRI in disease.
Keywords: CD89; autoimmunity; infection; inflammation; mucosa; neutrophil.
© 2020 van Gool and van Egmond.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures

Similar articles
-
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities.Front Immunol. 2019 Mar 22;10:553. doi: 10.3389/fimmu.2019.00553. eCollection 2019. Front Immunol. 2019. PMID: 30984170 Free PMC article. Review.
-
The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review.
-
Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives.Autoimmun Rev. 2013 Apr;12(6):666-9. doi: 10.1016/j.autrev.2012.10.011. Epub 2012 Nov 29. Autoimmun Rev. 2013. PMID: 23201915 Review.
-
The human immunoglobulin A Fc receptor FcαRI: a multifaceted regulator of mucosal immunity.Mucosal Immunol. 2011 Nov;4(6):612-24. doi: 10.1038/mi.2011.36. Epub 2011 Sep 21. Mucosal Immunol. 2011. PMID: 21937986 Review.
-
Anti-FcαRI Monoclonal Antibodies Resolve IgA Autoantibody-Mediated Disease.Front Immunol. 2022 Mar 15;13:732977. doi: 10.3389/fimmu.2022.732977. eCollection 2022. Front Immunol. 2022. PMID: 35371001 Free PMC article.
Cited by
-
Structural characterization of the Sel1-like repeat protein LceB from Legionella pneumophila.Protein Sci. 2024 Mar;33(3):e4889. doi: 10.1002/pro.4889. Protein Sci. 2024. PMID: 38160319 Free PMC article.
-
Autoimmunity in Pulmonary Arterial Hypertension: Evidence for Local Immunoglobulin Production.Front Cardiovasc Med. 2021 Sep 21;8:680109. doi: 10.3389/fcvm.2021.680109. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34621794 Free PMC article. Review.
-
Fcα Receptor-1-Activated Monocytes Promote B Lymphocyte Migration and IgA Isotype Switching.Int J Mol Sci. 2022 Sep 22;23(19):11132. doi: 10.3390/ijms231911132. Int J Mol Sci. 2022. PMID: 36232432 Free PMC article.
-
Advances in IgA glycosylation and its correlation with diseases.Front Chem. 2022 Sep 27;10:974854. doi: 10.3389/fchem.2022.974854. eCollection 2022. Front Chem. 2022. PMID: 36238099 Free PMC article. Review.
-
Proteomics and metabolomics analyses of Streptococcus agalactiae isolates from human and animal sources.Sci Rep. 2023 Nov 28;13(1):20980. doi: 10.1038/s41598-023-47976-y. Sci Rep. 2023. PMID: 38017083 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

