Cholinesterase inhibitory activity of highly functionalized fluorinated spiropyrrolidine heterocyclic hybrids
- PMID: 33424364
- PMCID: PMC7783807
- DOI: 10.1016/j.sjbs.2020.11.005
Cholinesterase inhibitory activity of highly functionalized fluorinated spiropyrrolidine heterocyclic hybrids
Abstract
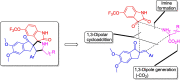
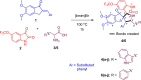
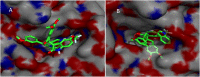
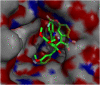
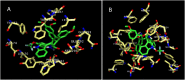
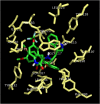
Two series of dimethoxyindanone imbedded novel fluorinated spiropyrrolidine heterocyclic hybrids were synthesized employing two different less explored azomethine ylides and were measured for their efficiency as inhibitors for Alzheimer's disease. Among the spiropyrrolidine heterocyclic hybrids, the indole based fluorinated compound with a methoxy substituent at the meta- position of the aryl ring exhibited the utmost potent AChE and BChE inhibitory activities with an IC50 of 1.97 ± 0.19 µM and 7.08 ± 0.20 µM respectively. The plausible mechanism of inhibition on ChE receptors was unveiled via molecular docking studies.
Keywords: AChE; Alzheimer’s disease; BChE; Cholinesterase inhibitory activity; Fluorinated spiroheterocyclic hybrids; Molecular docking simulation.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrids.Bioorg Med Chem Lett. 2020 Jan 15;30(2):126789. doi: 10.1016/j.bmcl.2019.126789. Epub 2019 Nov 7. Bioorg Med Chem Lett. 2020. PMID: 31753696
-
Ionic liquid-enabled synthesis, cholinesterase inhibitory activity, and molecular docking study of highly functionalized tetrasubstituted pyrrolidines.Bioorg Chem. 2018 Apr;77:263-268. doi: 10.1016/j.bioorg.2018.01.019. Epub 2018 Feb 3. Bioorg Chem. 2018. PMID: 29421701
-
Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: Stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study.Bioorg Chem. 2018 Sep;79:64-71. doi: 10.1016/j.bioorg.2018.04.025. Epub 2018 Apr 26. Bioorg Chem. 2018. PMID: 29723743
-
Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation.Bioorg Med Chem. 2019 Jun 15;27(12):2621-2628. doi: 10.1016/j.bmc.2019.03.058. Epub 2019 Mar 30. Bioorg Med Chem. 2019. PMID: 30952387
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
Cited by
-
Pyridine Scaffolds, Phenols and Derivatives of Azo Moiety: Current Therapeutic Perspectives.Molecules. 2021 Aug 11;26(16):4872. doi: 10.3390/molecules26164872. Molecules. 2021. PMID: 34443460 Free PMC article. Review.
-
Recent synthetic strategies of spiro-azetidin-2-one, -pyrrolidine, -indol(one) and -pyran derivatives-a review.RSC Adv. 2023 Nov 7;13(46):32786-32823. doi: 10.1039/d3ra06054c. eCollection 2023 Oct 31. RSC Adv. 2023. PMID: 37942448 Free PMC article. Review.
-
From tryptamine to the discovery of efficient multi-target directed ligands against cholinesterase-associated neurodegenerative disorders.Front Pharmacol. 2022 Nov 28;13:1036030. doi: 10.3389/fphar.2022.1036030. eCollection 2022. Front Pharmacol. 2022. PMID: 36518670 Free PMC article.
-
Design of Novel Enantiopure Dispirooxindolopyrrolidine-Piperidones as Promising Candidates toward COVID-19: Asymmetric Synthesis, Crystal Structure and In Silico Studies.Molecules. 2022 Jun 20;27(12):3945. doi: 10.3390/molecules27123945. Molecules. 2022. PMID: 35745069 Free PMC article.
-
Anti-aging and antioxidant of four traditional malaysian plants using simplex centroid mixture design approach.Saudi J Biol Sci. 2021 Dec;28(12):6711-6720. doi: 10.1016/j.sjbs.2021.07.048. Epub 2021 Jul 22. Saudi J Biol Sci. 2021. PMID: 34866970 Free PMC article.
References
-
- Ali M.A., Yar M.S., Hasan M.Z., Ahsan M.J., Pandian S. Design, synthesis and evaluation of novel 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenyl-3,4-substituted phenyl methanone analogues. Bioorg. Med. Chem. Lett. 2009;19:5075–5077. - PubMed
-
- Almansour A.I., Arumugam N., Kumar R.S., Kotresha D., Manohar T.S., Venketesh S. Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrid. Bioorg. Med. Chem. Lett. 2020;30:126789. - PubMed
-
- Basiri A., Murugaiyah V., Osman H., Kumar R.S., Kia Y., Ali M.A. Microwave assisted synthesis, cholinesterase enzymes inhibitory activities and molecular docking studies of new pyridopyrimidine derivatives. Bioorg. Med. Chem. 2013;21:3022–3031. - PubMed
-
- Basiri A., Murugaiyah V., Osman H., Kumar R.S., Kia Y., Awang K.B., Ali M.A. An expedient, ionic liquid mediated multi-component synthesis of novel piperidone grafted cholinesterase enzymes inhibitors and their molecular modeling study. Eur. J. Med. Chem. 2013;67:221–229. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

