Challenges and Perspectives for Therapeutic Targeting of Myeloproliferative Neoplasms
- PMID: 33403355
- PMCID: PMC7773330
- DOI: 10.1097/HS9.0000000000000516
Challenges and Perspectives for Therapeutic Targeting of Myeloproliferative Neoplasms
Abstract
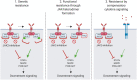
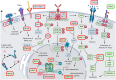
Myeloproliferative neoplasms (MPNs) are hematopoietic stem cell disorders with dysregulated myeloid blood cell production and propensity for transformation to acute myeloid leukemia, thrombosis, and bleeding. Acquired mutations in JAK2, MPL, and CALR converge on hyperactivation of Janus kinase 2 (JAK2) signaling as a central feature of MPN. Accordingly, JAK2 inhibitors have held promise for therapeutic targeting. After the JAK1/2 inhibitor ruxolitinib, similar JAK2 inhibitors as fedratinib are entering clinical use. While patients benefit with reduced splenomegaly and symptoms, disease-modifying effects on MPN clone size and clonal evolution are modest. Importantly, response to ruxolitinib may be lost upon treatment suggesting the MPN clone acquires resistance. Resistance mutations, as seen with other tyrosine kinase inhibitors, have not been described in MPN patients suggesting that functional processes reactivate JAK2 signaling. Compensatory signaling, which bypasses JAK2 inhibition, and other processes contribute to intrinsic resistance of MPN cells restricting efficacy of JAK2 inhibition overall. Combinations of JAK2 inhibition with pegylated interferon-α, a well-established therapy of MPN, B-cell lymphoma 2 inhibition, and others are in clinical development with the potential to enhance therapeutic efficacy. Novel single-agent approaches targeting other molecules than JAK2 are being investigated clinically. Special focus should be placed on myelofibrosis patients with anemia and thrombocytopenia, a delicate patient population at high need for options. The extending range of new treatment approaches will increase the therapeutic options for MPN patients. This calls for concomitant improvement of our insight into MPN biology to inform tailored therapeutic strategies for individual MPN patients.
Copyright © 2020 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures
Similar articles
-
SHP2 inhibition displays efficacy as a monotherapy and in combination with JAK2 inhibition in preclinical models of myeloproliferative neoplasms.Am J Hematol. 2024 Jun;99(6):1040-1055. doi: 10.1002/ajh.27282. Epub 2024 Mar 5. Am J Hematol. 2024. PMID: 38440831
-
The PIM inhibitor AZD1208 synergizes with ruxolitinib to induce apoptosis of ruxolitinib sensitive and resistant JAK2-V617F-driven cells and inhibit colony formation of primary MPN cells.Oncotarget. 2015 Nov 24;6(37):40141-57. doi: 10.18632/oncotarget.5653. Oncotarget. 2015. PMID: 26472029 Free PMC article.
-
Dual targeting of JAK2 and ERK interferes with the myeloproliferative neoplasm clone and enhances therapeutic efficacy.Leukemia. 2021 Oct;35(10):2875-2884. doi: 10.1038/s41375-021-01391-2. Epub 2021 Sep 3. Leukemia. 2021. PMID: 34480104 Free PMC article.
-
JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation.Blood Cancer J. 2022 Jan 26;12(1):13. doi: 10.1038/s41408-022-00609-5. Blood Cancer J. 2022. PMID: 35082276 Free PMC article. Review.
-
JAK2 in Myeloproliferative Neoplasms: Still a Protagonist.Pharmaceuticals (Basel). 2022 Jan 28;15(2):160. doi: 10.3390/ph15020160. Pharmaceuticals (Basel). 2022. PMID: 35215273 Free PMC article. Review.
Cited by
-
Associations of the circulating levels of cytokines with the risk of myeloproliferative neoplasms: a bidirectional mendelian-randomization study.BMC Cancer. 2024 Apr 26;24(1):531. doi: 10.1186/s12885-024-12301-x. BMC Cancer. 2024. PMID: 38671390 Free PMC article.
-
Interferons in the treatment of myeloproliferative neoplasms.Ther Adv Hematol. 2024 Feb 19;15:20406207241229588. doi: 10.1177/20406207241229588. eCollection 2024. Ther Adv Hematol. 2024. PMID: 38380373 Free PMC article. Review.
-
Calreticulin and JAK2V617F driver mutations induce distinct mitotic defects in myeloproliferative neoplasms.Sci Rep. 2024 Feb 2;14(1):2810. doi: 10.1038/s41598-024-53240-8. Sci Rep. 2024. PMID: 38308077 Free PMC article.
-
Jak2V617F Reversible Activation Shows Its Essential Requirement in Myeloproliferative Neoplasms.Cancer Discov. 2024 May 1;14(5):737-751. doi: 10.1158/2159-8290.CD-22-0952. Cancer Discov. 2024. PMID: 38230747 Free PMC article.
-
JAK2 inhibitors for the treatment of Philadelphia-negative myeloproliferative neoplasms: current status and future directions.Mol Divers. 2023 Nov 25. doi: 10.1007/s11030-023-10742-3. Online ahead of print. Mol Divers. 2023. PMID: 38006563 Review.
References
-
- Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017; 376:2168–2181. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. - PubMed
-
- Neubauer H, Cumano A, Müller M, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998; 93:397–409. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–1790. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
