Estimation of US SARS-CoV-2 Infections, Symptomatic Infections, Hospitalizations, and Deaths Using Seroprevalence Surveys
- PMID: 33399860
- PMCID: PMC7786245
- DOI: 10.1001/jamanetworkopen.2020.33706
Estimation of US SARS-CoV-2 Infections, Symptomatic Infections, Hospitalizations, and Deaths Using Seroprevalence Surveys
Abstract
Importance: Estimates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease burden are needed to help guide interventions.
Objective: To estimate the number of SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths in the US as of November 15, 2020.
Design, setting, and participants: In this cross-sectional study of respondents of all ages, data from 4 regional and 1 nationwide Centers for Disease Control and Prevention (CDC) seroprevalence surveys (April [n = 16 596], May, June, and July [n = 40 817], and August [n = 38 355]) were used to estimate infection underreporting multipliers and symptomatic underreporting multipliers. Community serosurvey data from randomly selected members of the general population were also used to validate the underreporting multipliers.
Main outcomes and measures: SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths. The median of underreporting multipliers derived from the 5 CDC seroprevalence surveys in the 10 states that participated in 2 or more surveys were applied to surveillance data of reported coronavirus disease 2019 (COVID-19) cases for 5 respective time periods to derive estimates of SARS-CoV-2 infections and symptomatic infections, which were summed to estimate SARS-CoV-2 infections and symptomatic infections in the US. Estimates of infections and symptomatic infections were combined with estimates of the hospitalization ratio and fatality ratio to derive estimates of SARS-CoV-2 hospitalizations and deaths. External validity of the surveys was evaluated with the April CDC survey by comparing results to 5 serosurveys (n = 22 118) that used random sampling of the general population. Internal validity of the multipliers from the 10 specific states was assessed in the August CDC survey by comparing multipliers from the 10 states to all states. A sensitivity analysis was conducted using the interquartile range of the multipliers to derive a high and low estimate of SARS-CoV-2 infections and symptomatic infections. The underreporting multipliers were then used to adjust the reported COVID-19 infections to estimate the full SARS-COV-2 disease burden.
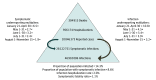
Results: Adjusting reported COVID-19 infections using underreporting multipliers derived from CDC seroprevalence studies in April (n = 16 596), May (n = 14 291), June (n = 14 159), July (n = 12 367), and August (n = 38 355), there were estimated medians of 46 910 006 (interquartile range [IQR], 38 192 705-60 814 748) SARS-CoV-2 infections, 28 122 752 (IQR, 23 014 957-36 438 592) symptomatic infections, 956 174 (IQR, 782 509-1 238 912) hospitalizations, and 304 915 (IQR, 248 253-395 296) deaths in the US through November 15, 2020. An estimated 14.3% (IQR, 11.6%-18.5%) of the US population were infected by SARS-CoV-2 as of mid-November 2020.
Conclusions and relevance: The SARS-CoV-2 disease burden may be much larger than reported COVID-19 cases owing to underreporting. Even after adjusting for underreporting, a substantial gap remains between the estimated proportion of the population infected and the proportion infected required to reach herd immunity. Additional seroprevalence surveys are needed to monitor the pandemic, including after the introduction of safe and efficacious vaccines.
Conflict of interest statement
Figures
Similar articles
-
Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020.JAMA Intern Med. 2021 Apr 1;181(4):450-460. doi: 10.1001/jamainternmed.2020.7976. JAMA Intern Med. 2021. PMID: 33231628 Free PMC article.
-
Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020-May 2021.JAMA. 2021 Oct 12;326(14):1400-1409. doi: 10.1001/jama.2021.15161. JAMA. 2021. PMID: 34473201 Free PMC article.
-
SARS-CoV-2 Infection Hospitalization Rate and Infection Fatality Rate Among the Non-Congregate Population in Connecticut.Am J Med. 2021 Jun;134(6):812-816.e2. doi: 10.1016/j.amjmed.2021.01.020. Epub 2021 Feb 20. Am J Med. 2021. PMID: 33617808 Free PMC article.
-
Systematic literature review of SARS-CoV-2 seroprevalence surveys in Canada through April 2021.IJID Reg. 2022 Sep;4:157-164. doi: 10.1016/j.ijregi.2022.07.010. Epub 2022 Jul 29. IJID Reg. 2022. PMID: 35919829 Free PMC article. Review.
-
Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review.Int J Infect Dis. 2020 Dec;101:314-322. doi: 10.1016/j.ijid.2020.10.011. Epub 2020 Oct 9. Int J Infect Dis. 2020. PMID: 33045429 Free PMC article. Review.
Cited by
-
Who Is at Risk of Poor Mental Health Following Coronavirus Disease-19 Outpatient Management?Front Med (Lausanne). 2022 Mar 14;9:792881. doi: 10.3389/fmed.2022.792881. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35360744 Free PMC article.
-
State-wide random seroprevalence survey of SARS-CoV-2 past infection in a southern US State, 2020.PLoS One. 2022 Apr 27;17(4):e0267322. doi: 10.1371/journal.pone.0267322. eCollection 2022. PLoS One. 2022. PMID: 35476717 Free PMC article.
-
Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers.Vaccines (Basel). 2022 Jul 22;10(8):1169. doi: 10.3390/vaccines10081169. Vaccines (Basel). 2022. PMID: 35893818 Free PMC article.
-
SARS-CoV-2 monitoring at three sewersheds of different scales and complexity demonstrates distinctive relationships between wastewater measurements and COVID-19 case data.Sci Total Environ. 2022 Apr 10;816:151534. doi: 10.1016/j.scitotenv.2021.151534. Epub 2021 Nov 13. Sci Total Environ. 2022. PMID: 34780821 Free PMC article.
-
Quarantine and testing strategies to ameliorate transmission due to travel during the COVID-19 pandemic: a modelling study.medRxiv [Preprint]. 2021 Dec 17:2021.04.25.21256082. doi: 10.1101/2021.04.25.21256082. medRxiv. 2021. Update in: Lancet Reg Health Eur. 2022 Mar;14:100304. doi: 10.1016/j.lanepe.2021.100304. PMID: 34729563 Free PMC article. Updated. Preprint.
References
-
- Centers for Disease Control and Prevention . Cases in the US. Accessed November 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
-
- Krantz SG, Rao ASRS. Level of underreporting including underdiagnosis before the first peak of COVID-19 in various countries: preliminary retrospective results based on wavelets and deterministic modeling. Infect Control Hosp Epidemiol. 2020;41(7):857-859. doi:10.1017/ice.2020.116 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . COVID-19 pandemic planning scenarios. Updated July 1, 2020. Accessed November 12, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios-h.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous