Poxviral Strategies to Overcome Host Cell Apoptosis
- PMID: 33374867
- PMCID: PMC7823800
- DOI: 10.3390/pathogens10010006
Poxviral Strategies to Overcome Host Cell Apoptosis
Abstract
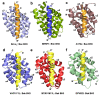
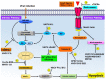
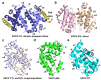
Apoptosis is a form of cellular suicide initiated either via extracellular (extrinsic apoptosis) or intracellular (intrinsic apoptosis) cues. This form of programmed cell death plays a crucial role in development and tissue homeostasis in multicellular organisms and its dysregulation is an underlying cause for many diseases. Intrinsic apoptosis is regulated by members of the evolutionarily conserved B-cell lymphoma-2 (Bcl-2) family, a family that consists of pro- and anti-apoptotic members. Bcl-2 genes have also been assimilated by numerous viruses including pox viruses, in particular the sub-family of chordopoxviridae, a group of viruses known to infect almost all vertebrates. The viral Bcl-2 proteins are virulence factors and aid the evasion of host immune defenses by mimicking the activity of their cellular counterparts. Viral Bcl-2 genes have proved essential for the survival of virus infected cells and structural studies have shown that though they often share very little sequence identity with their cellular counterparts, they have near-identical 3D structures. However, their mechanisms of action are varied. In this review, we examine the structural biology, molecular interactions, and detailed mechanism of action of poxvirus encoded apoptosis inhibitors and how they impact on host-virus interactions to ultimately enable successful infection and propagation of viral infections.
Keywords: Bcl-2; apoptosis; pox virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Bcl-2 Family in Host-Virus Interactions.Viruses. 2017 Oct 6;9(10):290. doi: 10.3390/v9100290. Viruses. 2017. PMID: 28984827 Free PMC article. Review.
-
The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms.Biomolecules. 2020 Jan 12;10(1):128. doi: 10.3390/biom10010128. Biomolecules. 2020. PMID: 31940915 Free PMC article. Review.
-
How do viruses control mitochondria-mediated apoptosis?Virus Res. 2015 Nov 2;209:45-55. doi: 10.1016/j.virusres.2015.02.026. Epub 2015 Mar 1. Virus Res. 2015. PMID: 25736565 Free PMC article. Review.
-
The Bcl-2 family: structures, interactions and targets for drug discovery.Apoptosis. 2015 Feb;20(2):136-50. doi: 10.1007/s10495-014-1051-7. Apoptosis. 2015. PMID: 25398535 Review.
-
To keep the host alive - the role of viral Bcl-2 proteins.Acta Virol. 2017;61(3):240-251. doi: 10.4149/av_2017_302. Acta Virol. 2017. PMID: 28854788 Review.
Cited by
-
Monkeypox: disease epidemiology, host immunity and clinical interventions.Nat Rev Immunol. 2022 Oct;22(10):597-613. doi: 10.1038/s41577-022-00775-4. Epub 2022 Sep 5. Nat Rev Immunol. 2022. PMID: 36064780 Free PMC article. Review.
-
Monkeypox virus infection of human astrocytes causes gasdermin B cleavage and pyroptosis.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2315653121. doi: 10.1073/pnas.2315653121. Epub 2024 Feb 12. Proc Natl Acad Sci U S A. 2024. PMID: 38346199 Free PMC article.
-
The prospective outcome of the monkeypox outbreak in 2022 and characterization of monkeypox disease immunobiology.Front Cell Infect Microbiol. 2023 Jul 18;13:1196699. doi: 10.3389/fcimb.2023.1196699. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37533932 Free PMC article.
-
Viral-mediated activation and inhibition of programmed cell death.PLoS Pathog. 2022 Aug 11;18(8):e1010718. doi: 10.1371/journal.ppat.1010718. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35951530 Free PMC article. Review.
-
Computational studies on searching potential phytochemicals against DNA polymerase activity of the monkeypox virus.J Tradit Complement Med. 2023 May 8;13(5):465-78. doi: 10.1016/j.jtcme.2023.04.002. Online ahead of print. J Tradit Complement Med. 2023. PMID: 37360910 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

