Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years
- PMID: 33372028
- PMCID: PMC7884998
- DOI: 10.1212/WNL.0000000000011242
Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years
Abstract
Objective: To test the hypothesis that immunotherapy prevents long-term disability in relapsing-remitting multiple sclerosis (MS), we modeled disability outcomes in 14,717 patients.
Methods: We studied patients from MSBase followed for ≥1 year, with ≥3 visits, ≥1 visit per year, and exposed to MS therapy, and a subset of patients with ≥15-year follow-up. Marginal structural models were used to compare the cumulative hazards of 12-month confirmed increase and decrease in disability, Expanded Disability Status Scale (EDSS) step 6, and the incidence of relapses between treated and untreated periods. Marginal structural models were continuously readjusted for patient age, sex, pregnancy, date, disease course, time from first symptom, prior relapse history, disability, and MRI activity.
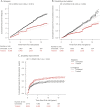
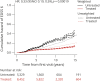
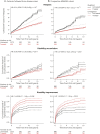
Results: A total of 14,717 patients were studied. During the treated periods, patients were less likely to experience relapses (hazard ratio 0.60, 95% confidence interval [CI] 0.43-0.82, p = 0.0016), worsening of disability (0.56, 0.38-0.82, p = 0.0026), and progress to EDSS step 6 (0.33, 0.19-0.59, p = 0.00019). Among 1,085 patients with ≥15-year follow-up, the treated patients were less likely to experience relapses (0.59, 0.50-0.70, p = 10-9) and worsening of disability (0.81, 0.67-0.99, p = 0.043).
Conclusion: Continued treatment with MS immunotherapies reduces disability accrual by 19%-44% (95% CI 1%-62%), the risk of need of a walking aid by 67% (95% CI 41%-81%), and the frequency of relapses by 40-41% (95% CI 18%-57%) over 15 years. This study provides evidence that disease-modifying therapies are effective in improving disability outcomes in relapsing-remitting MS over the long term.
Classification of evidence: This study provides Class IV evidence that, for patients with relapsing-remitting MS, long-term exposure to immunotherapy prevents neurologic disability.
© 2020 American Academy of Neurology.
Figures
Similar articles
-
Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study.Lancet Neurol. 2017 Apr;16(4):271-281. doi: 10.1016/S1474-4422(17)30007-8. Epub 2017 Feb 11. Lancet Neurol. 2017. PMID: 28209331
-
Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis.Brain. 2015 Nov;138(Pt 11):3275-86. doi: 10.1093/brain/awv260. Epub 2015 Sep 11. Brain. 2015. PMID: 26362907
-
Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis.JAMA. 2019 Jan 15;321(2):175-187. doi: 10.1001/jama.2018.20588. JAMA. 2019. PMID: 30644981 Free PMC article.
-
Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2. Cochrane Database Syst Rev. 2017. PMID: 28440858 Free PMC article. Review.
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD009882. doi: 10.1002/14651858.CD009882.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. PMID: 23235682 Updated. Review.
Cited by
-
Disease-modifying therapies and T1 hypointense lesions in patients with multiple sclerosis: A systematic review and meta-analysis.CNS Neurosci Ther. 2022 May;28(5):648-657. doi: 10.1111/cns.13815. Epub 2022 Feb 25. CNS Neurosci Ther. 2022. PMID: 35218155 Free PMC article.
-
Ponesimod in the Treatment of Relapsing Forms of Multiple Sclerosis: An Update on the Emerging Clinical Data.Degener Neurol Neuromuscul Dis. 2022 Mar 22;12:61-73. doi: 10.2147/DNND.S313825. eCollection 2022. Degener Neurol Neuromuscul Dis. 2022. PMID: 35356493 Free PMC article. Review.
-
An Atypical Presentation of Progressive Multiple Sclerosis in a Young Black Male.Cureus. 2023 Sep 18;15(9):e45496. doi: 10.7759/cureus.45496. eCollection 2023 Sep. Cureus. 2023. PMID: 37727844 Free PMC article.
-
Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper).Ther Adv Neurol Disord. 2021 Aug 18;14:17562864211039648. doi: 10.1177/17562864211039648. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 34422112 Free PMC article. Review.
-
Longitudinal humoral response in MS patients treated with cladribine tablets after receiving the second and third doses of SARS-CoV-2 mRNA vaccine.Mult Scler Relat Disord. 2022 Jul;63:103863. doi: 10.1016/j.msard.2022.103863. Epub 2022 May 10. Mult Scler Relat Disord. 2022. PMID: 35667316 Free PMC article.
References
-
- Coles AJ, Compston DA, Selmaj KW, et al. . Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–1801. - PubMed
-
- Polman CH, O'Connor PW, Havrdova E, et al. . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. - PubMed
-
- Kappos L, Radue E-W, O'Connor P, et al. . A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. - PubMed
-
- Hauser SL, Bar-Or A, Comi G, et al. . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
