Gene regulation by long non-coding RNAs and its biological functions
- PMID: 33353982
- PMCID: PMC7754182
- DOI: 10.1038/s41580-020-00315-9
Gene regulation by long non-coding RNAs and its biological functions
Erratum in
-
Author Correction: Gene regulation by long non-coding RNAs and its biological functions.Nat Rev Mol Cell Biol. 2021 Feb;22(2):159. doi: 10.1038/s41580-021-00330-4. Nat Rev Mol Cell Biol. 2021. PMID: 33420484 Free PMC article. No abstract available.
Abstract
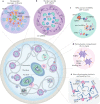
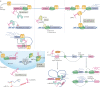
Evidence accumulated over the past decade shows that long non-coding RNAs (lncRNAs) are widely expressed and have key roles in gene regulation. Recent studies have begun to unravel how the biogenesis of lncRNAs is distinct from that of mRNAs and is linked with their specific subcellular localizations and functions. Depending on their localization and their specific interactions with DNA, RNA and proteins, lncRNAs can modulate chromatin function, regulate the assembly and function of membraneless nuclear bodies, alter the stability and translation of cytoplasmic mRNAs and interfere with signalling pathways. Many of these functions ultimately affect gene expression in diverse biological and physiopathological contexts, such as in neuronal disorders, immune responses and cancer. Tissue-specific and condition-specific expression patterns suggest that lncRNAs are potential biomarkers and provide a rationale to target them clinically. In this Review, we discuss the mechanisms of lncRNA biogenesis, localization and functions in transcriptional, post-transcriptional and other modes of gene regulation, and their potential therapeutic applications.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Role of mammalian long non-coding RNAs in normal and neuro oncological disorders.Genomics. 2021 Sep;113(5):3250-3273. doi: 10.1016/j.ygeno.2021.07.015. Epub 2021 Jul 21. Genomics. 2021. PMID: 34302945 Review.
-
Long noncoding RNAs: regulation, function and cancer.Biotechnol Genet Eng Rev. 2018 Oct;34(2):153-180. doi: 10.1080/02648725.2018.1471566. Epub 2018 Aug 3. Biotechnol Genet Eng Rev. 2018. PMID: 30071765 Review.
-
Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer.Genomics Proteomics Bioinformatics. 2016 Feb;14(1):42-54. doi: 10.1016/j.gpb.2015.09.006. Epub 2016 Feb 12. Genomics Proteomics Bioinformatics. 2016. PMID: 26883671 Free PMC article. Review.
-
Immunobiology of Long Noncoding RNAs.Annu Rev Immunol. 2017 Apr 26;35:177-198. doi: 10.1146/annurev-immunol-041015-055459. Epub 2017 Jan 11. Annu Rev Immunol. 2017. PMID: 28125358 Free PMC article. Review.
-
Roles for long non-coding RNAs in physiology and disease.Pflugers Arch. 2016 Jun;468(6):945-58. doi: 10.1007/s00424-016-1804-y. Epub 2016 Mar 5. Pflugers Arch. 2016. PMID: 26944276 Review.
Cited by
-
LncRNA FEZF1-AS1 promotes pulmonary fibrosis via up-regulating EZH2 and targeting miR-200c-3p to regulate the ZEB1 pathway.Sci Rep. 2024 Oct 30;14(1):26044. doi: 10.1038/s41598-024-74570-7. Sci Rep. 2024. PMID: 39472569 Free PMC article.
-
Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) lncRNA Conformational Dynamics in Complex with RNA-Binding Protein with Serine-Rich Domain 1 (RNPS1) in the Pan-cancer Splicing and Gene Expression.ACS Omega. 2024 Oct 3;9(41):42212-42226. doi: 10.1021/acsomega.4c04467. eCollection 2024 Oct 15. ACS Omega. 2024. PMID: 39431102 Free PMC article.
-
MicroRNA and Rare Human Diseases.Genes (Basel). 2024 Sep 25;15(10):1243. doi: 10.3390/genes15101243. Genes (Basel). 2024. PMID: 39457367 Free PMC article. Review.
-
Roles of lncRNAs related to the p53 network in breast cancer progression.Front Oncol. 2024 Oct 16;14:1453807. doi: 10.3389/fonc.2024.1453807. eCollection 2024. Front Oncol. 2024. PMID: 39479021 Free PMC article. Review.
-
Disrupted expression of long non-coding RNAs in the human oocyte: the possible epigenetic culprits leading to recurrent oocyte maturation arrest.J Assist Reprod Genet. 2022 Oct;39(10):2215-2225. doi: 10.1007/s10815-022-02596-9. Epub 2022 Aug 26. J Assist Reprod Genet. 2022. PMID: 36018477 Free PMC article.
References
-
- Wu H, Yang L, Chen LL. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33:540–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

