REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19
- PMID: 33332778
- PMCID: PMC7781102
- DOI: 10.1056/NEJMoa2035002
REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19
Abstract
Background: Recent data suggest that complications and death from coronavirus disease 2019 (Covid-19) may be related to high viral loads.
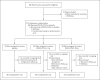
Methods: In this ongoing, double-blind, phase 1-3 trial involving nonhospitalized patients with Covid-19, we investigated two fully human, neutralizing monoclonal antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, used in a combined cocktail (REGN-COV2) to reduce the risk of the emergence of treatment-resistant mutant virus. Patients were randomly assigned (1:1:1) to receive placebo, 2.4 g of REGN-COV2, or 8.0 g of REGN-COV2 and were prospectively characterized at baseline for endogenous immune response against SARS-CoV-2 (serum antibody-positive or serum antibody-negative). Key end points included the time-weighted average change in viral load from baseline (day 1) through day 7 and the percentage of patients with at least one Covid-19-related medically attended visit through day 29. Safety was assessed in all patients.
Results: Data from 275 patients are reported. The least-squares mean difference (combined REGN-COV2 dose groups vs. placebo group) in the time-weighted average change in viral load from day 1 through day 7 was -0.56 log10 copies per milliliter (95% confidence interval [CI], -1.02 to -0.11) among patients who were serum antibody-negative at baseline and -0.41 log10 copies per milliliter (95% CI, -0.71 to -0.10) in the overall trial population. In the overall trial population, 6% of the patients in the placebo group and 3% of the patients in the combined REGN-COV2 dose groups reported at least one medically attended visit; among patients who were serum antibody-negative at baseline, the corresponding percentages were 15% and 6% (difference, -9 percentage points; 95% CI, -29 to 11). The percentages of patients with hypersensitivity reactions, infusion-related reactions, and other adverse events were similar in the combined REGN-COV2 dose groups and the placebo group.
Conclusions: In this interim analysis, the REGN-COV2 antibody cocktail reduced viral load, with a greater effect in patients whose immune response had not yet been initiated or who had a high viral load at baseline. Safety outcomes were similar in the combined REGN-COV2 dose groups and the placebo group. (Funded by Regeneron Pharmaceuticals and the Biomedical and Advanced Research and Development Authority of the Department of Health and Human Services; ClinicalTrials.gov number, NCT04425629.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Monoclonal Antibodies to Disrupt Progression of Early Covid-19 Infection.N Engl J Med. 2021 Jan 21;384(3):289-291. doi: 10.1056/NEJMe2034495. N Engl J Med. 2021. PMID: 33471984 Free PMC article. No abstract available.
Similar articles
-
SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19.N Engl J Med. 2021 Jan 21;384(3):229-237. doi: 10.1056/NEJMoa2029849. Epub 2020 Oct 28. N Engl J Med. 2021. PMID: 33113295 Free PMC article. Clinical Trial.
-
Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2021 Feb 16;325(7):632-644. doi: 10.1001/jama.2021.0202. JAMA. 2021. PMID: 33475701 Free PMC article. Clinical Trial.
-
REGN-COV2 antibody cocktail in patients with SARS-CoV-2: Observational study from a single institution in Japan.J Infect Chemother. 2022 Jul;28(7):943-947. doi: 10.1016/j.jiac.2022.03.029. Epub 2022 Apr 7. J Infect Chemother. 2022. PMID: 35414436 Free PMC article.
-
Melatonin and REGN-CoV2 combination as a vaccine adjuvant for Omicron variant of SARS-CoV-2.Mol Biol Rep. 2022 May;49(5):4061-4068. doi: 10.1007/s11033-022-07419-9. Epub 2022 Apr 7. Mol Biol Rep. 2022. PMID: 35389130 Free PMC article. Review.
-
Development and application of therapeutic antibodies against COVID-19.Int J Biol Sci. 2021 Apr 10;17(6):1486-1496. doi: 10.7150/ijbs.59149. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907512 Free PMC article. Review.
Cited by
-
Saving millions of lives but some resources squandered: emerging lessons from health research system pandemic achievements and challenges.Health Res Policy Syst. 2022 Sep 10;20(1):99. doi: 10.1186/s12961-022-00883-6. Health Res Policy Syst. 2022. PMID: 36088365 Free PMC article.
-
Treatment paradigms in Parkinson's Disease and Covid-19.Int Rev Neurobiol. 2022;165:135-171. doi: 10.1016/bs.irn.2022.03.002. Epub 2022 May 28. Int Rev Neurobiol. 2022. PMID: 36208898 Free PMC article.
-
Convalescent plasma to treat COVID-19: Following the Argentinian lead.Transfus Apher Sci. 2021 Jun;60(3):103161. doi: 10.1016/j.transci.2021.103161. Epub 2021 May 23. Transfus Apher Sci. 2021. PMID: 34045121 Free PMC article. Review. No abstract available.
-
A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019.Open Forum Infect Dis. 2022 Aug 8;9(8):ofac406. doi: 10.1093/ofid/ofac406. eCollection 2022 Aug. Open Forum Infect Dis. 2022. PMID: 36043180 Free PMC article.
-
An Update on SARS-CoV-2 Clinical Trial Results-What We Can Learn for the Next Pandemic.Int J Mol Sci. 2023 Dec 26;25(1):354. doi: 10.3390/ijms25010354. Int J Mol Sci. 2023. PMID: 38203525 Free PMC article. Review.
References
-
- Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020;584:425-429. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
