Physioxia Stimulates Extracellular Matrix Deposition and Increases Mechanical Properties of Human Chondrocyte-Derived Tissue-Engineered Cartilage
- PMID: 33282851
- PMCID: PMC7691651
- DOI: 10.3389/fbioe.2020.590743
Physioxia Stimulates Extracellular Matrix Deposition and Increases Mechanical Properties of Human Chondrocyte-Derived Tissue-Engineered Cartilage
Abstract
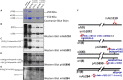
Cartilage tissue has been recalcitrant to tissue engineering approaches. In this study, human chondrocytes were formed into self-assembled cartilage sheets, cultured in physiologic (5%) and atmospheric (20%) oxygen conditions and underwent biochemical, histological and biomechanical analysis at 1- and 2-months. The results indicated that sheets formed at physiological oxygen tension were thicker, contained greater amounts of glycosaminoglycans (GAGs) and type II collagen, and had greater compressive and tensile properties than those cultured in atmospheric oxygen. In all cases, cartilage sheets stained throughout for extracellular matrix components. Type II-IX-XI collagen heteropolymer formed in the neo-cartilage and fibrils were stabilized by trivalent pyridinoline cross-links. Collagen cross-links were not significantly affected by oxygen tension but increased with time in culture. Physiological oxygen tension and longer culture periods both served to increase extracellular matrix components. The foremost correlation was found between compressive stiffness and the GAG to collagen ratio.
Keywords: articular cartilage; biomechanical testing; chondrocyte; chondrogenesis; collagen cross linking; hypoxia; tissue-engineered cartilage; type II collagen.
Copyright © 2020 Dennis, Whitney, Rai, Fernandes and Kean.
Figures


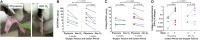


Similar articles
-
Synoviocyte Derived-Extracellular Matrix Enhances Human Articular Chondrocyte Proliferation and Maintains Re-Differentiation Capacity at Both Low and Atmospheric Oxygen Tensions.PLoS One. 2015 Jun 15;10(6):e0129961. doi: 10.1371/journal.pone.0129961. eCollection 2015. PLoS One. 2015. PMID: 26075742 Free PMC article.
-
Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity.Stem Cell Res Ther. 2016 Oct 20;7(1):154. doi: 10.1186/s13287-016-0419-8. Stem Cell Res Ther. 2016. PMID: 27765063 Free PMC article.
-
Disparate response of articular- and auricular-derived chondrocytes to oxygen tension.Connect Tissue Res. 2016 Jul;57(4):319-33. doi: 10.1080/03008207.2016.1182996. Epub 2016 Apr 29. Connect Tissue Res. 2016. PMID: 27128439 Free PMC article.
-
Articular cartilage: tissue design and chondrocyte-matrix interactions.Instr Course Lect. 1998;47:477-86. Instr Course Lect. 1998. PMID: 9571449 Review.
-
Glycosaminoglycan-Mediated Interactions in Articular, Auricular, Meniscal, and Nasal Cartilage.Tissue Eng Part B Rev. 2024 Apr 22. doi: 10.1089/ten.TEB.2023.0346. Online ahead of print. Tissue Eng Part B Rev. 2024. PMID: 38613808 Review.
Cited by
-
Proteomic-Based Analysis of Hypoxia- and Physioxia-Responsive Proteins and Pathways in Diffuse Large B-Cell Lymphoma.Cells. 2021 Aug 8;10(8):2025. doi: 10.3390/cells10082025. Cells. 2021. PMID: 34440794 Free PMC article.
-
Inverse Regulation of Cartilage Neogenesis at Physiologically Relevant Calcium Conditions by Human Articular Chondrocytes and Mesenchymal Stromal Cells.Cells. 2023 Jun 18;12(12):1659. doi: 10.3390/cells12121659. Cells. 2023. PMID: 37371129 Free PMC article.
-
Synoviocyte-Derived Extracellular Matrix and bFGF Speed Human Chondrocyte Proliferation While Maintaining Differentiation Potential.Front Bioeng Biotechnol. 2022 May 24;10:825005. doi: 10.3389/fbioe.2022.825005. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35685088 Free PMC article.
-
Micronutrient optimization for tissue engineered articular cartilage production of type II collagen.Front Bioeng Biotechnol. 2023 Jun 6;11:1179332. doi: 10.3389/fbioe.2023.1179332. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37346792 Free PMC article.
-
Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis.Healthcare (Basel). 2024 Aug 19;12(16):1648. doi: 10.3390/healthcare12161648. Healthcare (Basel). 2024. PMID: 39201206 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

