The armed oncolytic adenovirus ZD55-IL-24 eradicates melanoma by turning the tumor cells from the self-state into the nonself-state besides direct killing
- PMID: 33257647
- PMCID: PMC7705698
- DOI: 10.1038/s41419-020-03223-0
The armed oncolytic adenovirus ZD55-IL-24 eradicates melanoma by turning the tumor cells from the self-state into the nonself-state besides direct killing
Abstract
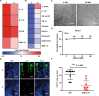
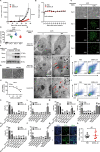
ZD55-IL-24 is similar but superior to the oncolytic adenovirus ONYX-015, yet the exact mechanism underlying the observed therapeutic effect is still not well understood. Here we sought to elucidate the underlying antitumor mechanism of ZD55-IL-24 in both immunocompetent and immunocompromised mouse model. We find that ZD55-IL-24 eradicates established melanoma in B16-bearing immunocompetent mouse model not through the classic direct killing pathway, but mainly through the indirect pathway of inducing systemic antitumor immunity. Inconsistent with the current prevailing view, our further results suggest that ZD55-IL-24 can induce antitumor immunity in B16-bearing immunocompetent mouse model in fact not due to its ability to lyse tumor cells and release the essential elements, such as tumor-associated antigens (TAAs), but due to its ability to put a "nonself" label in tumor cells and then turn the tumor cells from the "self" state into the "nonself" state without tumor cell death. The observed anti-melanoma efficacy of ZD55-IL-24 in B16-bearing immunocompetent mouse model was practically caused only by the viral vector. In addition, we also notice that ZD55-IL-24 can inhibit tumor growth in B16-bearing immunocompetent mouse model through inhibiting angiogenesis, despite it plays only a minor role. In contrast to B16-bearing immunocompetent mouse model, ZD55-IL-24 eliminates established melanoma in A375-bearing immunocompromised mouse model mainly through the classic direct killing pathway, but not through the antitumor immunity pathway and anti-angiogenesis pathway. These findings let us know ZD55-IL-24 more comprehensive and profound, and provide a sounder theoretical foundation for its future modification and drug development.
Conflict of interest statement
X.-Y.L., J.-F.G., and L.-Y.S. are inventors on a patent for the construction and application of ZD55-IL-24 (US Patent and Trademark Office, 20090117643A1). The other authors declare no competing financial interests.
Figures


Similar articles
-
Optimization of the Administration Strategy for the Armed Oncolytic Adenovirus ZD55-IL-24 in Both Immunocompromised and Immunocompetent Mouse Models.Hum Gene Ther. 2021 Dec;32(23-24):1481-1494. doi: 10.1089/hum.2021.036. Epub 2021 Jul 28. Hum Gene Ther. 2021. PMID: 34155929
-
Enhanced anti-melanoma efficacy through a combination of the armed oncolytic adenovirus ZD55-IL-24 and immune checkpoint blockade in B16-bearing immunocompetent mouse model.Cancer Immunol Immunother. 2021 Dec;70(12):3541-3555. doi: 10.1007/s00262-021-02946-z. Epub 2021 Apr 26. Cancer Immunol Immunother. 2021. PMID: 33903973 Free PMC article.
-
Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer.Hum Gene Ther. 2005 Jul;16(7):845-58. doi: 10.1089/hum.2005.16.845. Hum Gene Ther. 2005. PMID: 16000066
-
Targeting gene-virotherapy of cancer and its prosperity.Cell Res. 2006 Nov;16(11):879-86. doi: 10.1038/sj.cr.7310108. Cell Res. 2006. PMID: 17102812 Review.
-
Targeting gene-virotherapy of cancer.Cell Res. 2006 Jan;16(1):25-30. doi: 10.1038/sj.cr.7310005. Cell Res. 2006. Retraction in: Cell Res. 2006 Aug;16(8):740. doi: 10.1038/sj.cr.7310088 PMID: 16467873 Retracted. Review.
Cited by
-
Interleukins in cancer: from biology to therapy.Nat Rev Cancer. 2021 Aug;21(8):481-499. doi: 10.1038/s41568-021-00363-z. Epub 2021 Jun 3. Nat Rev Cancer. 2021. PMID: 34083781 Free PMC article. Review.
-
Immunotherapy: A new target for cancer cure (Review).Oncol Rep. 2023 May;49(5):100. doi: 10.3892/or.2023.8537. Epub 2023 Mar 31. Oncol Rep. 2023. PMID: 36999633 Free PMC article. Review.
-
Current development in adenoviral vectors for cancer immunotherapy.Mol Ther Oncolytics. 2021 Nov 20;23:571-581. doi: 10.1016/j.omto.2021.11.014. eCollection 2021 Dec 17. Mol Ther Oncolytics. 2021. PMID: 34938857 Free PMC article. Review.
-
Improved antitumor effectiveness of oncolytic HSV-1 viruses engineered with IL-15/IL-15Rα complex combined with oncolytic HSV-1-aPD1 targets colon cancer.Sci Rep. 2024 Oct 10;14(1):23671. doi: 10.1038/s41598-024-72888-w. Sci Rep. 2024. PMID: 39389985 Free PMC article.
-
Harnessing adenovirus in cancer immunotherapy: evoking cellular immunity and targeting delivery in cell-specific manner.Biomark Res. 2024 Mar 25;12(1):36. doi: 10.1186/s40364-024-00581-1. Biomark Res. 2024. PMID: 38528632 Free PMC article. Review.
References
-
- Liu X, Pestka S, Shi Y. Recent Advances in Cancer Research and Therapy. Beijing: Tsinghua University Press; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

