Ginsenoside Rg1 exerts neuroprotective effects in 3-nitropronpionic acid-induced mouse model of Huntington's disease via suppressing MAPKs and NF-κB pathways in the striatum
- PMID: 33214696
- PMCID: PMC8379213
- DOI: 10.1038/s41401-020-00558-4
Ginsenoside Rg1 exerts neuroprotective effects in 3-nitropronpionic acid-induced mouse model of Huntington's disease via suppressing MAPKs and NF-κB pathways in the striatum
Abstract
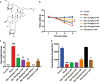
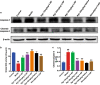
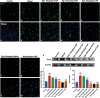
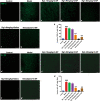
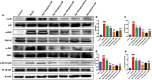
Huntington's disease (HD) is one of main neurodegenerative diseases, characterized by striatal atrophy, involuntary movements, and motor incoordination. Ginsenoside Rg1 (Rg1), an active ingredient in ginseng, possesses a variety of neuroprotective effects with low toxicity and side effects. In this study, we investigated the potential therapeutic effects of Rg1 in a mouse model of HD and explored the underlying mechanisms. HD was induced in mice by injection of 3-nitropropionic acid (3-NP, i.p.) for 4 days. From the first day of 3-NP injection, the mice were administered Rg1 (10, 20, 40 mg·kg-1, p.o.) for 5 days. We showed that oral pretreatment with Rg1 alleviated 3-NP-induced body weight loss and behavioral defects. Furthermore, pretreatment with Rg1 ameliorated 3-NP-induced neuronal loss and ultrastructural morphological damage in the striatum. Moreover, pretreatment with Rg1 reduced 3-NP-induced apoptosis and inhibited the activation of microglia, inflammatory mediators in the striatum. We revealed that Rg1 exerted neuroprotective effects by suppressing 3-NP-induced activation of the MAPKs and NF-κΒ signaling pathways in the striatum. Thus, our results suggest that Rg1 exerts therapeutic effects on 3-NP-induced HD mouse model via suppressing MAPKs and NF-κΒ signaling pathways. Rg1 may be served as a novel therapeutic option for HD.
Keywords: 3-nitropropionic acid; Huntington’s disease; MAPKs; NF-κB; ginsenoside Rg1; neuroprotective effects; striatum.
© 2020. CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Ethyl pyruvate ameliorates 3-nitropropionic acid-induced striatal toxicity through anti-neuronal cell death and anti-inflammatory mechanisms.Brain Behav Immun. 2014 May;38:151-65. doi: 10.1016/j.bbi.2014.01.015. Epub 2014 Feb 25. Brain Behav Immun. 2014. PMID: 24576481
-
Sulforaphane Ameliorates 3-Nitropropionic Acid-Induced Striatal Toxicity by Activating the Keap1-Nrf2-ARE Pathway and Inhibiting the MAPKs and NF-κB Pathways.Mol Neurobiol. 2016 May;53(4):2619-35. doi: 10.1007/s12035-015-9230-2. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26096705
-
Gintonin, a ginseng-derived ingredient, as a novel therapeutic strategy for Huntington's disease: Activation of the Nrf2 pathway through lysophosphatidic acid receptors.Brain Behav Immun. 2019 Aug;80:146-162. doi: 10.1016/j.bbi.2019.03.001. Epub 2019 Mar 7. Brain Behav Immun. 2019. PMID: 30853569
-
Protective Effect of Natural Products against Huntington's Disease: An Overview of Scientific Evidence and Understanding Their Mechanism of Action.ACS Chem Neurosci. 2021 Feb 3;12(3):391-418. doi: 10.1021/acschemneuro.0c00824. Epub 2021 Jan 21. ACS Chem Neurosci. 2021. PMID: 33475334 Review.
-
A clinical study and future prospects for bioactive compounds and semi-synthetic molecules in the therapies for Huntington's disease.Mol Neurobiol. 2024 Mar;61(3):1237-1270. doi: 10.1007/s12035-023-03604-4. Epub 2023 Sep 12. Mol Neurobiol. 2024. PMID: 37698833 Review.
Cited by
-
Pharmacological effects of natural medicine ginsenosides against Alzheimer's disease.Front Pharmacol. 2022 Nov 16;13:952332. doi: 10.3389/fphar.2022.952332. eCollection 2022. Front Pharmacol. 2022. PMID: 36467099 Free PMC article. Review.
-
Use of invertebrates to model chemically induced parkinsonism-symptoms.Biochem Soc Trans. 2023 Feb 27;51(1):435-445. doi: 10.1042/BST20221172. Biochem Soc Trans. 2023. PMID: 36645005 Free PMC article. Review.
-
Ginsenosides from Panax ginseng as Key Modulators of NF-κB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents.Int J Mol Sci. 2023 Mar 24;24(7):6119. doi: 10.3390/ijms24076119. Int J Mol Sci. 2023. PMID: 37047092 Free PMC article. Review.
-
Mechanistic insights on TLR-4 mediated inflammatory pathway in neurodegenerative diseases.Pharmacol Rep. 2024 Aug;76(4):679-692. doi: 10.1007/s43440-024-00613-5. Epub 2024 Jun 25. Pharmacol Rep. 2024. PMID: 38918327 Review.
-
Effects of Red ginseng on neuroinflammation in neurodegenerative diseases.J Ginseng Res. 2024 Jan;48(1):20-30. doi: 10.1016/j.jgr.2023.08.003. Epub 2023 Aug 29. J Ginseng Res. 2024. PMID: 38223824 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

