Downregulation of Interferon- γ Receptor Expression Endows Resistance to Anti-Programmed Death Protein 1 Therapy in Colorectal Cancer
- PMID: 33158943
- PMCID: PMC7745088
- DOI: 10.1124/jpet.120.000284
Downregulation of Interferon- γ Receptor Expression Endows Resistance to Anti-Programmed Death Protein 1 Therapy in Colorectal Cancer
Abstract
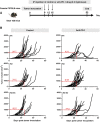
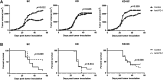
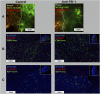
Immune checkpoint inhibitors have emerged as a frontline treatment of a variety of malignancies. However, only a subset of patients respond to these therapies, and many initial responders eventually develop resistance, leading to tumor relapse. Programmed death protein 1 is one of the checkpoint inhibitors that is expressed on activated T cells and suppresses the antitumor immune response when binding to its ligand, programmed death ligand 1, on tumor cells. Previous studies indicated that loss-of-function mutations in the IFN-γ pathway could result in acquired resistance to immune checkpoint inhibitors in human patients with cancer. Here, we investigated the effects of the IFN-γ receptor downexpression on the response to an anti-PD-1 antibody (αPD1) in a murine colorectal cancer model and the underlying mechanisms of resistance. IFN-γ receptor (IFNGR) 1 was knocked down in MC38 cells, a murine colon adenocarcinoma cell line using IFNGR1 short hairpin RNA (shRNA) lentiviral particles. Then, MC38 IFNGR1 knockdown (KD) cells and negative control (SC) cells were used in this study. In the C57BL/6 xenograft model, the KD tumor demonstrated resistance to αPD1 in comparison with SC cells. The observed treatment resistance might be associated with reduced tumor-infiltrating immune cells (TILs). When mixed, the resistant (KD) and control cells (SC) grew in spatially separated tumor areas, and αPD1 did not impact this pattern of spatial distribution. Our findings have proved that downregulation of the IFNGR1 endowed resistance to αPD1 and provided the potential mechanisms involving the TILs. SIGNIFICANCE STATEMENT: Immunological checkpoint blockades have achieved substantial efficacy in a variety of tumors. However, only a subset of patients respond to these therapies, and innate and acquired resistance is widely present. Our study found that the downregulation of the IFN-γ receptor caused resistance to an anti-PD-1 antibody in a murine colorectal cancer model associated with the reduced tumor-infiltrating lymphocytes. Our findings have substantial implications for improving the efficacy of checkpoint blockades.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
No author has an actual or perceived conflict of interest with the contents of this article.
Figures


Similar articles
-
Monocyte-derived APCs are central to the response of PD1 checkpoint blockade and provide a therapeutic target for combination therapy.J Immunother Cancer. 2020 Jul;8(2):e000588. doi: 10.1136/jitc-2020-000588. J Immunother Cancer. 2020. PMID: 32690667 Free PMC article.
-
Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice.Gastroenterology. 2020 Aug;159(2):609-623. doi: 10.1053/j.gastro.2020.03.074. Epub 2020 Apr 8. Gastroenterology. 2020. PMID: 32275969
-
CC-01 (chidamide plus celecoxib) modifies the tumor immune microenvironment and reduces tumor progression combined with immune checkpoint inhibitor.Sci Rep. 2022 Jan 20;12(1):1100. doi: 10.1038/s41598-022-05055-8. Sci Rep. 2022. PMID: 35058524 Free PMC article.
-
Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker.Cancer Med. 2020 Nov;9(21):8086-8121. doi: 10.1002/cam4.3410. Epub 2020 Sep 2. Cancer Med. 2020. PMID: 32875727 Free PMC article. Review.
-
The Role of Immune Checkpoint Inhibitors in Colorectal Adenocarcinoma.BioDrugs. 2020 Jun;34(3):349-362. doi: 10.1007/s40259-020-00420-3. BioDrugs. 2020. PMID: 32246441 Review.
Cited by
-
Utilizing a Proximity Dependent Labeling Strategy to Study Cancer-Immune Intercellular Interactions In Vitro and In Vivo.J Pharmacol Exp Ther. 2024 May 21;389(3):246-253. doi: 10.1124/jpet.123.001761. J Pharmacol Exp Ther. 2024. PMID: 37770200
-
N-glycosylation Regulates Intrinsic IFN-γ Resistance in Colorectal Cancer: Implications for Immunotherapy.Gastroenterology. 2023 Mar;164(3):392-406.e5. doi: 10.1053/j.gastro.2022.11.018. Epub 2022 Nov 17. Gastroenterology. 2023. PMID: 36402190 Free PMC article.
-
The Role of Inflammatory Cytokines in the Pathogenesis of Colorectal Carcinoma-Recent Findings and Review.Biomedicines. 2022 Jul 11;10(7):1670. doi: 10.3390/biomedicines10071670. Biomedicines. 2022. PMID: 35884974 Free PMC article. Review.
-
A Proximity-Dependent Biosensor System for Visualizing Cell-Cell Interactions Induced by Therapeutic Antibodies.J Pharm Sci. 2024 Mar;113(3):579-586. doi: 10.1016/j.xphs.2023.12.008. Epub 2023 Dec 14. J Pharm Sci. 2024. PMID: 38103691
-
Deregulation of interferon-gamma receptor 1 expression and its implications for lung adenocarcinoma progression.World J Clin Oncol. 2024 Feb 24;15(2):195-207. doi: 10.5306/wjco.v15.i2.195. World J Clin Oncol. 2024. PMID: 38455133 Free PMC article. Review.
References
-
- Callahan MK, Postow MA, Wolchok JD. (2016) Targeting T cell co-receptors for cancer therapy. Immunity 44:1069–1078. - PubMed
-
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al. (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928. - PubMed
-
- Chen DS, Mellman I. (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
