Emerging Roles of Downstream of Kinase 3 in Cell Signaling
- PMID: 33133079
- PMCID: PMC7550416
- DOI: 10.3389/fimmu.2020.566192
Emerging Roles of Downstream of Kinase 3 in Cell Signaling
Abstract
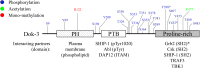
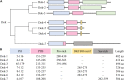
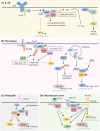
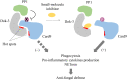
Downstream of kinase (Dok) 3 is a member of the Dok family of adaptor proteins known to regulate signaling pathways downstream of various immunoreceptors. As Dok-3 lacks intrinsic catalytic activity, it functions primarily as a molecular scaffold to facilitate the nucleation of protein complexes in a regulated manner and hence, achieve specificity in directing signaling cascades. Since its discovery, considerable progress has been made toward defining the role of Dok-3 in limiting B cell-receptor signaling. Nonetheless, Dok-3 has since been implicated in the signaling of Toll-like and C-type lectin receptors. Emerging data further demonstrate that Dok-3 can act both as an activator and inhibitor, in lymphoid and non-lymphoid cell types, suggesting Dok-3 involvement in a plethora of signal transduction pathways. In this review, we will focus on the structure and expression profile of Dok-3 and highlight its role during signal transduction in B cells, innate cells as well as in bone and lung tissues.
Keywords: B cells; Dok-3; adaptor; cell signaling; innate cells.
Copyright © 2020 Loh, Teo, Lim and Lam.
Figures
Similar articles
-
The roles of Dok family adapters in immunoreceptor signaling.Immunol Rev. 2009 Nov;232(1):273-85. doi: 10.1111/j.1600-065X.2009.00844.x. Immunol Rev. 2009. PMID: 19909370 Review.
-
Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling.J Immunol. 2002 May 1;168(9):4430-9. doi: 10.4049/jimmunol.168.9.4430. J Immunol. 2002. PMID: 11970986
-
Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling.J Exp Med. 2005 Feb 7;201(3):333-9. doi: 10.1084/jem.20041817. J Exp Med. 2005. PMID: 15699069 Free PMC article.
-
Dok-3 sequesters Grb2 and inhibits the Ras-Erk pathway downstream of protein-tyrosine kinases.Genes Cells. 2006 Feb;11(2):143-51. doi: 10.1111/j.1365-2443.2006.00926.x. Genes Cells. 2006. PMID: 16436051
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
Dok3 restrains neutrophil production of calprotectin during TLR4 sensing of SARS-CoV-2 spike protein.Front Immunol. 2022 Sep 12;13:996637. doi: 10.3389/fimmu.2022.996637. eCollection 2022. Front Immunol. 2022. PMID: 36172386 Free PMC article.
-
Adaptor molecules mediate negative regulation of macrophage inflammatory pathways: a closer look.Front Immunol. 2024 Feb 28;15:1355012. doi: 10.3389/fimmu.2024.1355012. eCollection 2024. Front Immunol. 2024. PMID: 38482001 Free PMC article. Review.
-
Neutrophils in the Pathogenesis of Rheumatic Diseases.Rheumatol Immunol Res. 2022 Oct 20;3(3):120-127. doi: 10.2478/rir-2022-0020. eCollection 2022 Oct. Rheumatol Immunol Res. 2022. PMID: 36788971 Free PMC article. Review.
-
The Caenorhabditis elegans protein SOC-3 permits an alternative mode of signal transduction by the EGL-15 FGF receptor.Dev Biol. 2024 Dec;516:183-195. doi: 10.1016/j.ydbio.2024.08.014. Epub 2024 Aug 21. Dev Biol. 2024. PMID: 39173814
-
Disrupting the Dok3-Card9 Interaction with Synthetic Peptides Enhances Antifungal Effector Functions of Human Neutrophils.Pharmaceutics. 2023 Jun 21;15(7):1780. doi: 10.3390/pharmaceutics15071780. Pharmaceutics. 2023. PMID: 37513967 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

