SARS-CoV-2 viral load is associated with increased disease severity and mortality
- PMID: 33127906
- PMCID: PMC7603483
- DOI: 10.1038/s41467-020-19057-5
SARS-CoV-2 viral load is associated with increased disease severity and mortality
Abstract
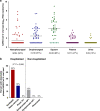
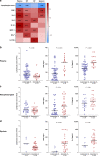
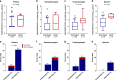
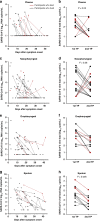
The relationship between SARS-CoV-2 viral load and risk of disease progression remains largely undefined in coronavirus disease 2019 (COVID-19). Here, we quantify SARS-CoV-2 viral load from participants with a diverse range of COVID-19 disease severity, including those requiring hospitalization, outpatients with mild disease, and individuals with resolved infection. We detected SARS-CoV-2 plasma RNA in 27% of hospitalized participants, and 13% of outpatients diagnosed with COVID-19. Amongst the participants hospitalized with COVID-19, we report that a higher prevalence of detectable SARS-CoV-2 plasma viral load is associated with worse respiratory disease severity, lower absolute lymphocyte counts, and increased markers of inflammation, including C-reactive protein and IL-6. SARS-CoV-2 viral loads, especially plasma viremia, are associated with increased risk of mortality. Our data show that SARS-CoV-2 viral loads may aid in the risk stratification of patients with COVID-19, and therefore its role in disease pathogenesis should be further explored.
Conflict of interest statement
J.Z.L. has consulted for Abbvie and Jan Biotech. G.A. is the founder of Seromyx Inc. All other authors declare no competing interests.
Figures
Similar articles
-
RNAemia Corresponds to Disease Severity and Antibody Response in Hospitalized COVID-19 Patients.Viruses. 2020 Sep 18;12(9):1045. doi: 10.3390/v12091045. Viruses. 2020. PMID: 32962125 Free PMC article.
-
SARS-CoV-2 RNAemia in a Healthy Blood Donor 40 Days After Respiratory Illness Resolution.Ann Intern Med. 2020 Nov 17;173(10):853-854. doi: 10.7326/L20-0725. Epub 2020 Jul 17. Ann Intern Med. 2020. PMID: 32678685 Free PMC article. No abstract available.
-
Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study.EBioMedicine. 2020 Oct;60:102999. doi: 10.1016/j.ebiom.2020.102999. Epub 2020 Sep 16. EBioMedicine. 2020. PMID: 32950003 Free PMC article.
-
Association of elevated inflammatory markers and severe COVID-19: A meta-analysis.Medicine (Baltimore). 2020 Nov 20;99(47):e23315. doi: 10.1097/MD.0000000000023315. Medicine (Baltimore). 2020. PMID: 33217868 Free PMC article. Review.
-
Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review.Clin Biochem. 2020 Jul;81:1-8. doi: 10.1016/j.clinbiochem.2020.05.012. Epub 2020 May 27. Clin Biochem. 2020. PMID: 32473151 Free PMC article. Review.
Cited by
-
From bedside to bench: regulation of host factors in SARS-CoV-2 infection.Exp Mol Med. 2021 Apr;53(4):483-494. doi: 10.1038/s12276-021-00595-x. Epub 2021 Apr 7. Exp Mol Med. 2021. PMID: 33828231 Free PMC article. Review.
-
Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses.J Pediatr. 2020 Dec;227:45-52.e5. doi: 10.1016/j.jpeds.2020.08.037. Epub 2020 Aug 20. J Pediatr. 2020. PMID: 32827525 Free PMC article.
-
Use of ivermectin in the treatment of Covid-19: A pilot trial.Toxicol Rep. 2021;8:505-510. doi: 10.1016/j.toxrep.2021.03.003. Epub 2021 Mar 9. Toxicol Rep. 2021. Retraction in: Toxicol Rep. 2022 May 02;9:1023. doi: 10.1016/j.toxrep.2022.04.009 PMID: 33723507 Free PMC article. Retracted.
-
A stronger antibody response in increased disease severity of SARS-CoV-2.BMC Infect Dis. 2024 Jan 2;24(1):17. doi: 10.1186/s12879-023-08923-4. BMC Infect Dis. 2024. PMID: 38166763 Free PMC article.
-
Promising Adjunct Medicines in the Protocol of COVID-19 Clinical Trial.Adv Pharm Bull. 2022 Aug;12(4):641-644. doi: 10.34172/apb.2022.067. Epub 2021 Oct 6. Adv Pharm Bull. 2022. PMID: 36415639 Free PMC article.
References
-
- He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med.10.1038/s41591-020-0869-5 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- U19 AI135995/AI/NIAID NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
- U01 CK000490/CK/NCEZID CDC HHS/United States
- K08 HL141694/HL/NHLBI NIH HHS/United States
- K08 DK114563/DK/NIDDK NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- U01CK000490/ACL/ACL HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 HL142093/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

