To TRIM the Immunity: From Innate to Adaptive Immunity
- PMID: 33117334
- PMCID: PMC7578260
- DOI: 10.3389/fimmu.2020.02157
To TRIM the Immunity: From Innate to Adaptive Immunity
Abstract
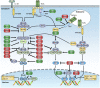
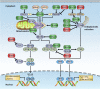
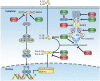
The tripartite motif (TRIM) proteins have been intensively studied as essential modulators in various biological processes, especially in regulating a wide range of signaling pathways involved in immune responses. Most TRIM proteins have E3 ubiquitin ligase activity, mediating polyubiquitination of target proteins. Emerging evidence demonstrates that TRIM proteins play important roles in innate immunity by regulating pattern recognition receptors, vital adaptor proteins, kinases, and transcription factors in innate immune signaling pathways. Additionally, the critical roles of TRIM proteins in adaptive immunity, especially in T cell development and activation, are increasingly appreciated. In this review, we aim to summarize the studies on TRIMs in both innate and adaptive immunity, focusing on their E3 ubiquitin ligase functions in pattern recognition receptor signaling pathways and T cell functions, shedding light on the developing new strategies for modulating innate and adaptive immune responses against invading pathogens and avoiding autoimmunity.
Keywords: adaptive immunity; innate immunity; signal; tripartite motif; ubiquitination.
Copyright © 2020 Yang, Gu, Zhang and Hu.
Figures

Similar articles
-
To TRIM or not to TRIM: the balance of host-virus interactions mediated by the ubiquitin system.J Gen Virol. 2019 Dec;100(12):1641-1662. doi: 10.1099/jgv.0.001341. J Gen Virol. 2019. PMID: 31661051 Free PMC article.
-
Tripartite motif (TRIM) proteins roles in the regulation of immune system responses: Focus on autoimmune diseases.Exp Cell Res. 2025 Jan 15;444(2):114379. doi: 10.1016/j.yexcr.2024.114379. Epub 2024 Dec 10. Exp Cell Res. 2025. PMID: 39667699 Review.
-
TRIM proteins and diseases.J Biochem. 2017 Feb 1;161(2):135-144. doi: 10.1093/jb/mvw087. J Biochem. 2017. PMID: 28069866 Review.
-
TRIMming Type I Interferon-Mediated Innate Immune Response in Antiviral and Antitumor Defense.Viruses. 2021 Feb 11;13(2):279. doi: 10.3390/v13020279. Viruses. 2021. PMID: 33670221 Free PMC article. Review.
-
The Roles of TRIMs in Antiviral Innate Immune Signaling.Front Cell Infect Microbiol. 2021 Mar 15;11:628275. doi: 10.3389/fcimb.2021.628275. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791238 Free PMC article. Review.
Cited by
-
It's a TRIM-endous view from the top: the varied roles of TRIpartite Motif proteins in brain development and disease.Front Mol Neurosci. 2023 Dec 5;16:1287257. doi: 10.3389/fnmol.2023.1287257. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38115822 Free PMC article. Review.
-
Identification of Nonsynonymous SNPs in Immune-Related Genes Associated with Pneumonia Severity in Pigs.Genes (Basel). 2024 Aug 21;15(8):1103. doi: 10.3390/genes15081103. Genes (Basel). 2024. PMID: 39202462 Free PMC article.
-
Multifaceted roles for STAT3 in gammaherpesvirus latency revealed through in vivo B cell knockout models.mBio. 2024 Feb 14;15(2):e0299823. doi: 10.1128/mbio.02998-23. Epub 2024 Jan 3. mBio. 2024. PMID: 38170993 Free PMC article.
-
Duck TRIM29 negatively regulates type I IFN production by targeting MAVS.Front Immunol. 2023 Jan 6;13:1016214. doi: 10.3389/fimmu.2022.1016214. eCollection 2022. Front Immunol. 2023. PMID: 36685538 Free PMC article.
-
Effects of sustained hyperprolactinemia in late gestation on the mammary parenchymal tissue transcriptome of gilts.BMC Genomics. 2023 Jan 24;24(1):40. doi: 10.1186/s12864-023-09136-4. BMC Genomics. 2023. PMID: 36694114 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

