Low-density Lipoprotein-Cholesterol Lowering Strategies for Prevention of Atherosclerotic Cardiovascular Disease: Focus on siRNA Treatment Targeting PCSK9 (Inclisiran)
- PMID: 33089390
- PMCID: PMC7578129
- DOI: 10.1007/s11886-020-01427-6
Low-density Lipoprotein-Cholesterol Lowering Strategies for Prevention of Atherosclerotic Cardiovascular Disease: Focus on siRNA Treatment Targeting PCSK9 (Inclisiran)
Abstract
Purpose of review: The aim of low-density lipoprotein-cholesterol (LDL-C) lowering therapies is to safely achieve a consistent and long-term reduction in exposure of the vasculature to atherogenic lipoproteins in order to reduce the risk of atherosclerotic cardiovascular (CV) disease and the associated CV events, such as myocardial infarctions and ischemic strokes. This review summarizes the concept and clinical development of a novel molecular approach to efficiently lower LDL-C, a synthetic small interfering ribonucleic acid (siRNA)-inclisiran-directed against proprotein convertase subtilisin-kexin type 9 (PCSK9).
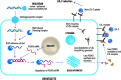
Recent findings: The understanding of genes regulating atherogenic lipoproteins and their causal role in the development of atherosclerotic CV disease has substantially advanced over the past years. This has opened the possibility for development of molecular therapies targeting these atherogenic lipoproteins, in particular by RNA-targeted treatment approaches. The most advanced clinical development program is the siRNA-treatment targeting PCSK9 (inclisiran), involving more than 4000 patients in clinical studies. Phase 1 and 2 studies have identified the dose of 300 mg inclisiran for efficient LDL-C lowering. Most recently, three phase 3 studies demonstrated that a regimen of inclisiran every 6 months was feasible and reduced LDL-C by approximately 50% in patients at high or very high CV risk or with familial hypercholesterolemia. Adverse events were similar in the inclisiran and the placebo groups, except for more frequent transient injection site reactions with inclisiran than with placebo. siRNA therapy targeting PCSK9 (inclisiran) applied twice a year efficiently reduced LDL-C by approximately 50% and was safe in recent phase 3 studies. The effects of this treatment on CV outcome are currently further assessed in a large ongoing CV outcome trial.
Keywords: Coronary disease; Hypercholesterolemia; LDL-C; PCSK9; siRNA.
Conflict of interest statement
David Sinning has received lecture honoraria or advisory honoraria from Amgen, Sanofi, Hexal, Daiichi-Sankyo, and Berlin-Chemie. Ulf Landmesser has received lecture or advisory honoraria from Amgen, Sanofi, Novartis, Medicines Company, Bayer, and Berlin-Chemie.
Figures
Similar articles
-
Inclisiran: A Review in Hypercholesterolemia.Am J Cardiovasc Drugs. 2023 Mar;23(2):219-230. doi: 10.1007/s40256-023-00568-7. Epub 2023 Mar 4. Am J Cardiovasc Drugs. 2023. PMID: 36869996 Review.
-
Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran.J Am Heart Assoc. 2024 Mar 19;13(6):e032031. doi: 10.1161/JAHA.123.032031. Epub 2024 Mar 8. J Am Heart Assoc. 2024. PMID: 38456415 Free PMC article. Review.
-
The clinical effects of inclisiran, a first-in-class LDL-C lowering siRNA therapy, on the LDL-C levels in Chinese patients with hypercholesterolemia.J Clin Lipidol. 2023 May-Jun;17(3):392-400. doi: 10.1016/j.jacl.2023.04.010. Epub 2023 Apr 29. J Clin Lipidol. 2023. PMID: 37164838 Clinical Trial.
-
Effect of an siRNA Therapeutic Targeting PCSK9 on Atherogenic Lipoproteins: Prespecified Secondary End Points in ORION 1.Circulation. 2018 Sep 25;138(13):1304-1316. doi: 10.1161/CIRCULATIONAHA.118.034710. Circulation. 2018. PMID: 29735484 Clinical Trial.
-
Inclisiran: A First-in-Class siRNA Therapy for Lowering Low-Density Lipoprotein Cholesterol.Ann Pharmacother. 2023 Mar;57(3):317-324. doi: 10.1177/10600280221105169. Epub 2022 Jun 30. Ann Pharmacother. 2023. PMID: 35775133 Review.
Cited by
-
Potential of Phage Display Antibody Technology for Cardiovascular Disease Immunotherapy.J Cardiovasc Transl Res. 2022 Apr;15(2):360-380. doi: 10.1007/s12265-021-10169-x. Epub 2021 Aug 31. J Cardiovasc Transl Res. 2022. PMID: 34467463 Review.
-
[Primary disorders of lipid metabolism: their place in current dyslipidemia guidelines and treatment innovations].Inn Med (Heidelb). 2023 Sep;64(9):895-906. doi: 10.1007/s00108-023-01524-y. Epub 2023 Jun 6. Inn Med (Heidelb). 2023. PMID: 37280381 Review. German.
-
Inflammation and Cardiovascular Disease: The Future.Eur Cardiol. 2021 May 17;16:e20. doi: 10.15420/ecr.2020.50. eCollection 2021 Feb. Eur Cardiol. 2021. PMID: 34093741 Free PMC article. Review.
-
PCSK9 Inhibitor Wars: How Does Inclisiran Fit in with Current Monoclonal Antibody Inhibitor Therapy? Considerations for Patient Selection.Curr Cardiol Rep. 2022 Nov;24(11):1657-1667. doi: 10.1007/s11886-022-01782-6. Epub 2022 Sep 10. Curr Cardiol Rep. 2022. PMID: 36087240 Free PMC article. Review.
-
Treating Cardiovascular Disease with Liver Genome Engineering.Curr Atheroscler Rep. 2022 Feb;24(2):75-84. doi: 10.1007/s11883-022-00986-z. Epub 2022 Mar 1. Curr Atheroscler Rep. 2022. PMID: 35230602 Free PMC article. Review.
References
-
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388–2398. - PubMed
-
- Levy D. Combating the epidemic of heart disease. JAMA. 2012;308(24):2624–2625. - PubMed
-
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

