ATR addiction in multiple myeloma: synthetic lethal approaches exploiting established therapies
- PMID: 33054085
- PMCID: PMC7556682
- DOI: 10.3324/haematol.2018.215210
ATR addiction in multiple myeloma: synthetic lethal approaches exploiting established therapies
Abstract
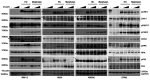
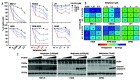
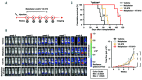
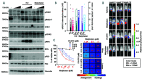
Therapeutic strategies designed to tinker with cancer cell DNA damage response have led to the widespread use of PARP inhibitors for BRCA1/2-mutated cancers. In the haematological cancer multiple myeloma, we sought to identify analogous synthetic lethality mechanisms that could be leveraged upon established cancer treatments. The combination of ATR inhibition using the compound VX-970 with a drug eliciting interstrand cross-links, melphalan, was tested in in vitro, ex vivo, and most notably in vivo models. Cell proliferation, induction of apoptosis, tumor growth and animal survival were assessed. The combination of ATM inhibition with a drug triggering double strand breaks, doxorucibin, was also probed. We found that ATR inhibition is strongly synergistic with melphalan, even in resistant cells. The combination was dramatically effective in targeting myeloma primary patient cells and cell lines reducing cell proliferation and inducing apoptosis. The combination therapy significantly reduced tumor burden and prolonged survival in animal models. Conversely, ATM inhibition only marginally impacted on myeloma cell survival, even in combination with doxorucibin at high doses. These results indicate that myeloma cells extensively rely on ATR, but not on ATM, for DNA repair. Our findings posit that adding an ATR inhibitor such as VX-970 to established therapeutic regimens may provide a remarkably broad benefit to myeloma patients.
Figures


Similar articles
-
An inverse switch in DNA base excision and strand break repair contributes to melphalan resistance in multiple myeloma cells.PLoS One. 2013;8(2):e55493. doi: 10.1371/journal.pone.0055493. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405159 Free PMC article.
-
PJ34, a poly(ADP-ribose) polymerase (PARP) inhibitor, reverses melphalan-resistance and inhibits repair of DNA double-strand breaks by targeting the FA/BRCA pathway in multidrug resistant multiple myeloma cell line RPMI8226/R.Int J Oncol. 2015 Jan;46(1):223-32. doi: 10.3892/ijo.2014.2726. Epub 2014 Oct 23. Int J Oncol. 2015. PMID: 25351371
-
A novel BCMA PBD-ADC with ATM/ATR/WEE1 inhibitors or bortezomib induce synergistic lethality in multiple myeloma.Leukemia. 2020 Aug;34(8):2150-2162. doi: 10.1038/s41375-020-0745-9. Epub 2020 Feb 14. Leukemia. 2020. PMID: 32060401 Free PMC article.
-
Targeting ATM and ATR for cancer therapeutics: Inhibitors in clinic.Drug Discov Today. 2023 Aug;28(8):103662. doi: 10.1016/j.drudis.2023.103662. Epub 2023 Jun 10. Drug Discov Today. 2023. PMID: 37302542 Review.
-
Roles of ATM and ATR in DNA double strand breaks and replication stress.Prog Biophys Mol Biol. 2021 Aug;163:109-119. doi: 10.1016/j.pbiomolbio.2021.03.007. Epub 2021 Apr 20. Prog Biophys Mol Biol. 2021. PMID: 33887296 Review.
Cited by
-
Comprehensive genomic analysis of refractory multiple myeloma reveals a complex mutational landscape associated with drug resistance and novel therapeutic vulnerabilities.Haematologica. 2022 Aug 1;107(8):1891-1901. doi: 10.3324/haematol.2021.279360. Haematologica. 2022. PMID: 35045690 Free PMC article.
-
A drug repurposing strategy for overcoming human multiple myeloma resistance to standard-of-care treatment.Cell Death Dis. 2022 Mar 4;13(3):203. doi: 10.1038/s41419-022-04651-w. Cell Death Dis. 2022. PMID: 35246527 Free PMC article.
-
Targeting the DNA damage response in hematological malignancies.Front Oncol. 2024 Jan 29;14:1307839. doi: 10.3389/fonc.2024.1307839. eCollection 2024. Front Oncol. 2024. PMID: 38347838 Free PMC article. Review.
-
Genomic Instability and Replicative Stress in Multiple Myeloma: The Final Curtain?Cancers (Basel). 2021 Dec 22;14(1):25. doi: 10.3390/cancers14010025. Cancers (Basel). 2021. PMID: 35008191 Free PMC article. Review.
-
Progression of monoclonal gammopathy of undetermined significance to multiple myeloma is associated with enhanced translational quality control and overall loss of surface antigens.J Transl Med. 2024 Jun 7;22(1):548. doi: 10.1186/s12967-024-05345-x. J Transl Med. 2024. PMID: 38849800 Free PMC article.
References
-
- Puigvert JC, Sanjiv K, Helleday T. Targeting DNA repair, DNA metabolism and replication stress as anti-cancer strategies. FEBS J. 2016; 283(2):232-245. - PubMed
-
- Iglehart JD, Silver DP. Synthetic lethality - a new direction in cancer-drug development. N Engl J Med. 2009; 361(2):189-191. - PubMed
-
- Kaelin WG. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5(9):689-698. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, et al. . Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADPribose) polymerase. Nature. 2005; 434(7035):913-917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

