A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes
- PMID: 33036983
- PMCID: PMC7557000
- DOI: 10.1126/sciadv.aaz9124
A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes
Abstract
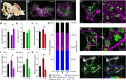
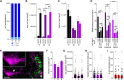
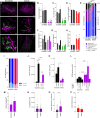
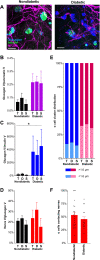
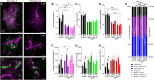
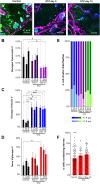
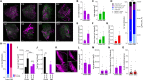
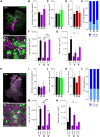
Understanding the detailed anatomy of the endocrine pancreas, its innervation, and the remodeling that occurs in diabetes can provide new insights into metabolic disease. Using tissue clearing and whole-organ imaging, we identified the 3D associations between islets and innervation. This technique provided detailed quantification of α and β cell volumes and pancreatic nerve fibers, their distribution and heterogeneity in healthy tissue, canonical mouse models of diabetes, and samples from normal and diabetic human pancreata. Innervation was highly enriched in the mouse endocrine pancreas, with regional differences. Islet nerve density was increased in nonobese diabetic mice, in mice treated with streptozotocin, and in pancreata of human donors with type 2 diabetes. Nerve contacts with β cells were preserved in diabetic mice and humans. In summary, our whole-organ assessment allows comprehensive examination of islet characteristics and their innervation and reveals dynamic regulation of islet innervation in diabetes.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

Similar articles
-
Human pancreatic capillaries and nerve fibers persist in type 1 diabetes despite beta cell loss.Am J Physiol Endocrinol Metab. 2023 Mar 1;324(3):E251-E267. doi: 10.1152/ajpendo.00246.2022. Epub 2023 Jan 25. Am J Physiol Endocrinol Metab. 2023. PMID: 36696598 Free PMC article.
-
Recovery of pancreatic beta cells in response to long-term normoglycemia after pancreas or islet transplantation in severely streptozotocin diabetic adult rats.Pancreas. 2001 Aug;23(2):186-96. doi: 10.1097/00006676-200108000-00009. Pancreas. 2001. PMID: 11484921
-
Structural and functional polarisation of human pancreatic beta cells in islets from organ donors with and without type 2 diabetes.Diabetologia. 2021 Mar;64(3):618-629. doi: 10.1007/s00125-020-05345-8. Epub 2021 Jan 5. Diabetologia. 2021. PMID: 33399909 Free PMC article.
-
Unravelling innervation of pancreatic islets.Diabetologia. 2022 Jul;65(7):1069-1084. doi: 10.1007/s00125-022-05691-9. Epub 2022 Mar 29. Diabetologia. 2022. PMID: 35348820 Free PMC article. Review.
-
Imaging of the islet neural network.Diabetes Obes Metab. 2014 Sep;16 Suppl 1:77-86. doi: 10.1111/dom.12342. Diabetes Obes Metab. 2014. PMID: 25200300 Review.
Cited by
-
Schwann Cells in Digestive System Disorders.Cells. 2022 Feb 28;11(5):832. doi: 10.3390/cells11050832. Cells. 2022. PMID: 35269454 Free PMC article. Review.
-
Neuroimmune regulation in the pancreas.Fundam Res. 2022 Aug 5;4(2):201-205. doi: 10.1016/j.fmre.2022.08.001. eCollection 2024 Mar. Fundam Res. 2022. PMID: 38933519 Free PMC article. Review.
-
The rheumatoid arthritis drug auranofin lowers leptin levels and exerts antidiabetic effects in obese mice.Cell Metab. 2022 Dec 6;34(12):1932-1946.e7. doi: 10.1016/j.cmet.2022.09.019. Epub 2022 Oct 14. Cell Metab. 2022. PMID: 36243005 Free PMC article.
-
Mesoscopic Optical Imaging of the Pancreas-Revisiting Pancreatic Anatomy and Pathophysiology.Front Endocrinol (Lausanne). 2021 Mar 4;12:633063. doi: 10.3389/fendo.2021.633063. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33746904 Free PMC article. Review.
-
Human pancreatic capillaries and nerve fibers persist in type 1 diabetes despite beta cell loss.Am J Physiol Endocrinol Metab. 2023 Mar 1;324(3):E251-E267. doi: 10.1152/ajpendo.00246.2022. Epub 2023 Jan 25. Am J Physiol Endocrinol Metab. 2023. PMID: 36696598 Free PMC article.
References
-
- Ahrén B., Autonomic regulation of islet hormone secretion—Implications for health and disease. Diabetologia 43, 393–410 (2000). - PubMed
-
- J. Gerald, J. Taborsky, Handbook of Physiology (Oxford Univ. Press, American Physiological Society, 2001).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

