Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients
- PMID: 33033173
- PMCID: PMC8050884
- DOI: 10.1126/sciimmunol.abe5511
Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients
Abstract
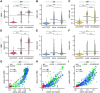
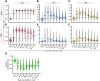
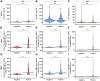
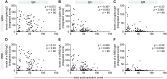
While the antibody response to SARS-CoV-2 has been extensively studied in blood, relatively little is known about the antibody response in saliva and its relationship to systemic antibody levels. Here, we profiled by enzyme-linked immunosorbent assays (ELISAs) IgG, IgA and IgM responses to the SARS-CoV-2 spike protein (full length trimer) and its receptor-binding domain (RBD) in serum and saliva of acute and convalescent patients with laboratory-diagnosed COVID-19 ranging from 3-115 days post-symptom onset (PSO), compared to negative controls. Anti-SARS-CoV-2 antibody responses were readily detected in serum and saliva, with peak IgG levels attained by 16-30 days PSO. Longitudinal analysis revealed that anti-SARS-CoV-2 IgA and IgM antibodies rapidly decayed, while IgG antibodies remained relatively stable up to 105 days PSO in both biofluids. Lastly, IgG, IgM and to a lesser extent IgA responses to spike and RBD in the serum positively correlated with matched saliva samples. This study confirms that serum and saliva IgG antibodies to SARS-CoV-2 are maintained in the majority of COVID-19 patients for at least 3 months PSO. IgG responses in saliva may serve as a surrogate measure of systemic immunity to SARS-CoV-2 based on their correlation with serum IgG responses.
Copyright © 2020, American Association for the Advancement of Science.
Figures


Similar articles
-
Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients.Sci Immunol. 2020 Oct 8;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367. Sci Immunol. 2020. PMID: 33033172 Free PMC article.
-
Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2.J Clin Microbiol. 2020 May 26;58(6):e00461-20. doi: 10.1128/JCM.00461-20. Print 2020 May 26. J Clin Microbiol. 2020. PMID: 32229605 Free PMC article.
-
Antibody kinetics and serologic profiles of SARS-CoV-2 infection using two serologic assays.PLoS One. 2020 Oct 22;15(10):e0240395. doi: 10.1371/journal.pone.0240395. eCollection 2020. PLoS One. 2020. PMID: 33091042 Free PMC article.
-
Antibody Response After SARS-CoV-2 Infection and Implications for Immunity : A Rapid Living Review.Ann Intern Med. 2021 Jun;174(6):811-821. doi: 10.7326/M20-7547. Epub 2021 Mar 16. Ann Intern Med. 2021. PMID: 33721517 Free PMC article. Review.
-
Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review.Rev Med Virol. 2021 Mar;31(2):e2162. doi: 10.1002/rmv.2162. Epub 2020 Sep 23. Rev Med Virol. 2021. PMID: 32964627 Free PMC article. Review.
Cited by
-
Intramuscular vaccination against SARS-CoV-2 transiently induces neutralizing IgG rather than IgA in the saliva.Front Immunol. 2024 Feb 5;15:1330864. doi: 10.3389/fimmu.2024.1330864. eCollection 2024. Front Immunol. 2024. PMID: 38375482 Free PMC article.
-
Minding the margins: Evaluating the impact of COVID-19 among Latinx and Black communities with optimal qualitative serological assessment tools.PLoS One. 2024 Jul 25;19(7):e0307568. doi: 10.1371/journal.pone.0307568. eCollection 2024. PLoS One. 2024. PMID: 39052608 Free PMC article.
-
Infectious virus shedding duration reflects secretory IgA antibody response latency after SARS-CoV-2 infection.Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2314808120. doi: 10.1073/pnas.2314808120. Epub 2023 Dec 22. Proc Natl Acad Sci U S A. 2023. PMID: 38134196 Free PMC article.
-
SARS-CoV-2 seroprevalence and associated factors among people living with HIV in Sierra Leone.Immun Inflamm Dis. 2024 Jul;12(7):e1338. doi: 10.1002/iid3.1338. Immun Inflamm Dis. 2024. PMID: 38990142 Free PMC article.
-
Affinity Sensors for the Diagnosis of COVID-19.Micromachines (Basel). 2021 Apr 2;12(4):390. doi: 10.3390/mi12040390. Micromachines (Basel). 2021. PMID: 33918184 Free PMC article. Review.
References
-
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T. H. O., Chromikova V., McMahon M., Jiang K., Arunkumar G. A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D. S., Lugo L. A., Kojic E. M., Stoever J., Liu S. T. H., Cunningham-Rundles C., Felgner P. L., Moran T., García-Sastre A., Caplivski D., Cheng A. C., Kedzierska K., Vapalahti O., Hepojoki J. M., Simon V., Krammer F., A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26, 1033–1036 (2020). 10.1038/s41591-020-0913-5 - DOI - PMC - PubMed
-
- Long Q. X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F., Wang D.-Q., Hu Y., Ren J.-H., Tang N., Xu Y.-Y., Yu L.-H., Mo Z., Gong F., Zhang X.-L., Tian W.-G., Hu L., Zhang X.-X., Xiang J.-L., Du H.-X., Liu H.-W., Lang C.-H., Luo X.-H., Wu S.-B., Cui X.-P., Zhou Z., Zhu M.-M., Wang J., Xue C.-J., Li X.-F., Wang L., Li Z.-J., Wang K., Niu C.-C., Yang Q.-J., Tang X.-J., Zhang Y., Liu X.-M., Li J.-J., Zhang D.-C., Zhang F., Liu P., Yuan J., Li Q., Hu J.-L., Chen J., Huang A.-L., Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020). 10.1038/s41591-020-0897-1 - DOI - PubMed
-
- Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D. R., Raut R., Markmann A., Cornaby C., Bartelt L., Weiss S., Park Y., Edwards C. E., Weimer E., Scherer E. M., Rouphael N., Edupuganti S., Weiskopf D., Tse L. V., Hou Y. J., Margolis D., Sette A., Collins M. H., Schmitz J., Baric R. S., de Silva A. M., The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 5, eabc8413 (2020). 10.1126/sciimmunol.abc8413 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

