Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
- PMID: 33031764
- PMCID: PMC7535623
- DOI: 10.1016/S0140-6736(20)32013-4
Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Abstract
Background: Lopinavir-ritonavir has been proposed as a treatment for COVID-19 on the basis of in vitro activity, preclinical studies, and observational studies. Here, we report the results of a randomised trial to assess whether lopinavir-ritonavir improves outcomes in patients admitted to hospital with COVID-19.
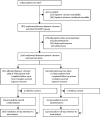
Methods: In this randomised, controlled, open-label, platform trial, a range of possible treatments was compared with usual care in patients admitted to hospital with COVID-19. The trial is underway at 176 hospitals in the UK. Eligible and consenting patients were randomly allocated to either usual standard of care alone or usual standard of care plus lopinavir-ritonavir (400 mg and 100 mg, respectively) by mouth for 10 days or until discharge (or one of the other RECOVERY treatment groups: hydroxychloroquine, dexamethasone, or azithromycin) using web-based simple (unstratified) randomisation with allocation concealment. Randomisation to usual care was twice that of any of the active treatment groups (eg, 2:1 in favour of usual care if the patient was eligible for only one active group, 2:1:1 if the patient was eligible for two active groups). The primary outcome was 28-day all-cause mortality. Analyses were done on an intention-to-treat basis in all randomly assigned participants. The trial is registered with ISRCTN, 50189673, and ClinicalTrials.gov, NCT04381936.
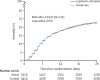
Findings: Between March 19, 2020, and June 29, 2020, 1616 patients were randomly allocated to receive lopinavir-ritonavir and 3424 patients to receive usual care. Overall, 374 (23%) patients allocated to lopinavir-ritonavir and 767 (22%) patients allocated to usual care died within 28 days (rate ratio 1·03, 95% CI 0·91-1·17; p=0·60). Results were consistent across all prespecified subgroups of patients. We observed no significant difference in time until discharge alive from hospital (median 11 days [IQR 5 to >28] in both groups) or the proportion of patients discharged from hospital alive within 28 days (rate ratio 0·98, 95% CI 0·91-1·05; p=0·53). Among patients not on invasive mechanical ventilation at baseline, there was no significant difference in the proportion who met the composite endpoint of invasive mechanical ventilation or death (risk ratio 1·09, 95% CI 0·99-1·20; p=0·092).
Interpretation: In patients admitted to hospital with COVID-19, lopinavir-ritonavir was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death. These findings do not support the use of lopinavir-ritonavir for treatment of patients admitted to hospital with COVID-19.
Funding: Medical Research Council and National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Antiviral monotherapy for hospitalised patients with COVID-19 is not enough.Lancet. 2020 Oct 24;396(10259):1310-1311. doi: 10.1016/S0140-6736(20)32078-X. Epub 2020 Oct 5. Lancet. 2020. PMID: 33031761 Free PMC article. No abstract available.
Similar articles
-
Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet. 2021 Feb 13;397(10274):605-612. doi: 10.1016/S0140-6736(21)00149-5. Epub 2021 Feb 2. Lancet. 2021. PMID: 33545096 Free PMC article. Clinical Trial.
-
Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet Respir Med. 2021 Dec;9(12):1419-1426. doi: 10.1016/S2213-2600(21)00435-5. Epub 2021 Oct 18. Lancet Respir Med. 2021. PMID: 34672950 Free PMC article. Clinical Trial.
-
Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet Diabetes Endocrinol. 2023 Dec;11(12):905-914. doi: 10.1016/S2213-8587(23)00253-X. Epub 2023 Oct 18. Lancet Diabetes Endocrinol. 2023. PMID: 37865101 Free PMC article. Clinical Trial.
-
Umifenovir in hospitalized moderate to severe COVID-19 patients: A randomized clinical trial.Int Immunopharmacol. 2021 Oct;99:107969. doi: 10.1016/j.intimp.2021.107969. Epub 2021 Jul 10. Int Immunopharmacol. 2021. PMID: 34273635 Free PMC article. Review.
-
Nirmatrelvir combined with ritonavir for preventing and treating COVID-19.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD015395. doi: 10.1002/14651858.CD015395.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Nov 30;11:CD015395. doi: 10.1002/14651858.CD015395.pub3 PMID: 36126225 Free PMC article. Updated. Review.
Cited by
-
Clinical features and prognostic factors of intensive and non-intensive 1014 COVID-19 patients: an experience cohort from Alahsa, Saudi Arabia.Eur J Med Res. 2021 May 24;26(1):47. doi: 10.1186/s40001-021-00517-7. Eur J Med Res. 2021. PMID: 34030733 Free PMC article.
-
In silico investigation of potential small molecule inhibitors of the SARS-CoV-2 nsp10-nsp16 methyltransferase complex.Chem Phys Lett. 2021 Jul;774:138618. doi: 10.1016/j.cplett.2021.138618. Epub 2021 Apr 9. Chem Phys Lett. 2021. PMID: 33850334 Free PMC article.
-
An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19.Clin Microbiol Infect. 2021 Dec;27(12):1826-1837. doi: 10.1016/j.cmi.2021.05.020. Epub 2021 May 26. Clin Microbiol Infect. 2021. PMID: 34048876 Free PMC article. Clinical Trial.
-
Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection.J Biomol Struct Dyn. 2022 Oct;40(16):7367-7380. doi: 10.1080/07391102.2021.1901144. Epub 2021 Mar 18. J Biomol Struct Dyn. 2022. PMID: 33734021 Free PMC article.
-
How to use COVID-19 antiviral drugs in patients with chronic kidney disease.Front Pharmacol. 2023 Feb 9;14:1053814. doi: 10.3389/fphar.2023.1053814. eCollection 2023. Front Pharmacol. 2023. PMID: 36843922 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

