Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis
- PMID: 33026597
- PMCID: PMC7538847
- DOI: 10.1186/s13613-020-00740-1
Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis
Abstract
Background: Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Despite treatment being in line with current guidelines, mortality remains high in those with septic shock. Intravenous immunoglobulins represent a promising therapy to modulate both the pro- and anti-inflammatory processes and can contribute to the elimination of pathogens. In this context, there is evidence of the benefits of immunoglobulin M (IgM)- and immunoglobulin A (IgA)-enriched immunoglobulin therapy for sepsis. This manuscript aims to summarize current relevant data to provide expert opinions on best practice for the use of an IgM- and IgA-enriched immunoglobulin (Pentaglobin) in adult patients with sepsis.
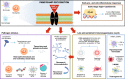
Main text: Sepsis patients with hyperinflammation and patients with immunosuppression may benefit most from treatment with IgM- and IgA-enriched immunoglobulin (Pentaglobin). Patients with hyperinflammation present with phenotypes that manifest throughout the body, whilst the clinical characteristics of immunosuppression are less clear. Potential biomarkers for hyperinflammation include elevated procalcitonin, interleukin-6, endotoxin activity and C-reactive protein, although thresholds for these are not well-defined. Convenient biomarkers for identifying patients in a stage of immune-paralysis are still matter of debate, though human leukocyte antigen-antigen D related expression on monocytes, lymphocyte count and viral reactivation have been proposed. The timing of treatment is potentially more critical for treatment efficacy in patients with hyperinflammation compared with patients who are in an immunosuppressed stage. Due to the lack of evidence, definitive dosage recommendations for either population cannot be made, though we suggest that patients with hyperinflammation should receive an initial bolus at a rate of up to 0.6 mL (30 mg)/kg/h for 6 h followed by a continuous maintenance rate of 0.2 mL (10 mg)/kg/hour for ≥ 72 h (total dose ≥ 0.9 g/kg). For immunosuppressed patients, dosage is more conservative (0.2 mL [10 mg]/kg/h) for ≥ 72 h, without an initial bolus (total dose ≥ 0.72 g/kg).
Conclusions: Two distinct populations that may benefit most from Pentaglobin therapy are described in this review. However, further clinical evidence is required to strengthen support for the recommendations given here regarding timing, duration and dosage of treatment.
Keywords: Hyperinflammation; IgM- and IgA-enriched immunoglobulin; Immunoglobulin; Immunosuppression; Pentaglobin; Sepsis.
Conflict of interest statement
AN: research funds, speaker honoraria and travel reimbursement from Biotest AG, CytoSorbents Europe, ThermoFisher Scientific. GB: declares that he has no competing interests. DKM: speaker honoraria from Biotest AG. EM: speaker, consultant and/or advisory board member honoraria from Astellas, AstraZeneca, Basilea, Bayer Vital, Biosyn Arzneimittel, Biotest AG, Fresenius Medical Care, GE Healthcare, Gilead Sciences, Janssen–Cilag, Merck Sharp & Dohme, Merck, Novartis, Pfizer, Sanofi–Aventis, Wyeth. MG: speaker and/or advisory board member honoraria from Amomed, BioMerieux, Biotest AG, Estor, Merck Sharp & Dohme, Nordic Pharma, NovoNordisk, Orion Pharma, Pfizer, Shinogi Europe, Thermofisher.
Figures
Similar articles
-
Endotoxin concentration in neutropenic patients with suspected gram-negative sepsis: correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins.Antimicrob Agents Chemother. 1992 Oct;36(10):2139-46. doi: 10.1128/AAC.36.10.2139. Antimicrob Agents Chemother. 1992. PMID: 1444293 Free PMC article.
-
Polyvalent immunoglobulin significantly attenuated the formation of IL-1β in Escherichia coli-induced sepsis in pigs.Immunobiology. 2013 May;218(5):683-9. doi: 10.1016/j.imbio.2012.08.268. Epub 2012 Aug 9. Immunobiology. 2013. PMID: 22947599
-
[The effect of pentaglobin therapy on prognosis in patients with severe sepsis].Ulus Travma Derg. 2001 Oct;7(4):219-23. Ulus Travma Derg. 2001. PMID: 11705075 Clinical Trial. Turkish.
-
Use of Intravenous Immunoglobulins in Sepsis Therapy-A Clinical View.Int J Mol Sci. 2020 Aug 3;21(15):5543. doi: 10.3390/ijms21155543. Int J Mol Sci. 2020. PMID: 32756325 Free PMC article. Review.
-
The use of IgM-enriched immunoglobulin in adult patients with sepsis.J Crit Care. 2018 Oct;47:30-35. doi: 10.1016/j.jcrc.2018.06.005. Epub 2018 Jun 3. J Crit Care. 2018. PMID: 29886064 Review.
Cited by
-
Treatment of donor-specific antibody-mediated rejection after heart transplantation by IgM-enriched human immunoglobulin.ESC Heart Fail. 2021 Aug;8(4):3413-3417. doi: 10.1002/ehf2.13409. Epub 2021 May 10. ESC Heart Fail. 2021. PMID: 33969938 Free PMC article.
-
IgM-enriched immunoglobulin as adjuvant therapy for heart transplant after infection of left ventricular assist devices.ESC Heart Fail. 2022 Oct;9(5):3630-3635. doi: 10.1002/ehf2.14074. Epub 2022 Jul 19. ESC Heart Fail. 2022. PMID: 35854478 Free PMC article.
-
The Functional Role of IgA in the IgM/IgA-Enriched Immunoglobulin Preparation Trimodulin.Biomedicines. 2021 Dec 3;9(12):1828. doi: 10.3390/biomedicines9121828. Biomedicines. 2021. PMID: 34944644 Free PMC article.
-
"Impact of pentaglobin in severe COVID 19 pneumonia- a prospective study.".Int Immunopharmacol. 2021 Oct;99:107968. doi: 10.1016/j.intimp.2021.107968. Epub 2021 Jul 10. Int Immunopharmacol. 2021. PMID: 34304002 Free PMC article.
-
A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases.Front Immunol. 2023 Mar 27;14:1127339. doi: 10.3389/fimmu.2023.1127339. eCollection 2023. Front Immunol. 2023. PMID: 37051237 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

