Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/ BRCA2
- PMID: 33008098
- PMCID: PMC7650720
- DOI: 10.3390/cancers12102834
Gene Panel Tumor Testing in Ovarian Cancer Patients Significantly Increases the Yield of Clinically Actionable Germline Variants beyond BRCA1/ BRCA2
Abstract
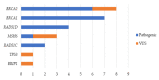
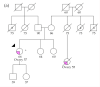
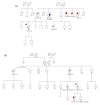
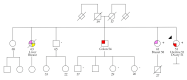
Since the approval of PARP inhibitors for the treatment of high-grade serous ovarian cancer, in addition to cancer risk assessment, BRCA1 and BRCA2 genetic testing also has therapeutic implications (germline and somatic variants) and should be offered to these patients at diagnosis, irrespective of family history. However, variants in other genes besides BRCA1 and BRCA2 are associated with ovarian cancer predisposition, which would be missed by a genetic testing aimed only at indication for PARP inhibitor treatment. In this study, we aimed to evaluate the yield of clinically actionable germline variants using next-generation sequencing of a customized panel of 10 genes for the analysis of formalin-fixed paraffin-embedded samples from 96 ovarian carcinomas, a strategy that allows the detection of both somatic and germline variants in a single test. In addition to 13.7% of deleterious germline BRCA1/BRCA2 carriers, we identified 7.4% additional patients with pathogenic germline variants in other genes predisposing for ovarian cancer, namely RAD51C, RAD51D, and MSH6, representing 35% of all pathogenic germline variants. We conclude that the strategy of reflex gene-panel tumor testing enables the identification of clinically actionable germline variants in a significantly higher proportion of ovarian cancer patients, which may be valuable information in patients with advanced disease that have run out of approved therapeutic options. Furthermore, this approach increases the chance to make available genetic counseling, presymptomatic genetic testing, and gynecological cancer prophylaxis to female relatives who turn out to be healthy carriers of deleterious germline variants.
Keywords: clinically actionable alterations; genetic predisposition; multi-gene panel; next generation sequencing; ovarian cancer; tumor testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Tumor Testing for Somatic and Germline BRCA1/BRCA2 Variants in Ovarian Cancer Patients in the Context of Strong Founder Effects.Front Oncol. 2020 Jul 31;10:1318. doi: 10.3389/fonc.2020.01318. eCollection 2020. Front Oncol. 2020. PMID: 32850417 Free PMC article.
-
Targeted molecular profiling of epithelial ovarian cancer from Italian BRCA wild-type patients with a BRCA and PARP pathways gene panel.Exp Mol Pathol. 2022 Oct;128:104833. doi: 10.1016/j.yexmp.2022.104833. Epub 2022 Sep 20. Exp Mol Pathol. 2022. PMID: 36165864
-
BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing.Oncotarget. 2016 Jan 12;7(2):1076-83. doi: 10.18632/oncotarget.6834. Oncotarget. 2016. PMID: 26745875 Free PMC article.
-
Twenty Years of BRCA1 and BRCA2 Molecular Analysis at MMCI - Current Developments for the Classification of Variants.Klin Onkol. 2019 Summer;32(Supplementum2):51-71. doi: 10.14735/amko2019S51. Klin Onkol. 2019. PMID: 31409081 Review. English.
-
Guidance Statement On BRCA1/2 Tumor Testing in Ovarian Cancer Patients.Semin Oncol. 2017 Jun;44(3):187-197. doi: 10.1053/j.seminoncol.2017.08.004. Epub 2017 Sep 15. Semin Oncol. 2017. PMID: 29248130 Review.
Cited by
-
Current Applications and Challenges of Next-Generation Sequencing in Plasma Circulating Tumour DNA of Ovarian Cancer.Biology (Basel). 2024 Jan 31;13(2):88. doi: 10.3390/biology13020088. Biology (Basel). 2024. PMID: 38392306 Free PMC article. Review.
-
Next Generation Sequencing of Tumor and Matched Plasma Samples: Identification of Somatic Variants in ctDNA From Ovarian Cancer Patients.Front Oncol. 2021 Sep 30;11:754094. doi: 10.3389/fonc.2021.754094. eCollection 2021. Front Oncol. 2021. PMID: 34660321 Free PMC article.
-
Clinical characteristics and survival analysis of Chinese ovarian cancer patients with RAD51D germline mutations.BMC Cancer. 2022 Dec 21;22(1):1337. doi: 10.1186/s12885-022-10456-z. BMC Cancer. 2022. PMID: 36544182 Free PMC article.
-
Germline RAD51C and RAD51D Mutations in High-Risk Chinese Breast and/or Ovarian Cancer Patients and Families.J Pers Med. 2024 Aug 16;14(8):866. doi: 10.3390/jpm14080866. J Pers Med. 2024. PMID: 39202057 Free PMC article.
-
Using Portuguese BRCA pathogenic variation as a model to study the impact of human admixture on human health.BMC Genomics. 2024 Apr 27;25(1):416. doi: 10.1186/s12864-024-10311-4. BMC Genomics. 2024. PMID: 38671360 Free PMC article.
References
-
- Morgan R.J., Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Behbakht K., Chen L.-M., Copeland L., Crispens M.A., DeRosa M., Dorigo O., et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016;14:1134–1163. doi: 10.6004/jnccn.2016.0122. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

