Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults
- PMID: 32991794
- PMCID: PMC7556339
- DOI: 10.1056/NEJMoa2028436
Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults
Abstract
Background: Testing of vaccine candidates to prevent infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an older population is important, since increased incidences of illness and death from coronavirus disease 2019 (Covid-19) have been associated with an older age.
Methods: We conducted a phase 1, dose-escalation, open-label trial of a messenger RNA vaccine, mRNA-1273, which encodes the stabilized prefusion SARS-CoV-2 spike protein (S-2P) in healthy adults. The trial was expanded to include 40 older adults, who were stratified according to age (56 to 70 years or ≥71 years). All the participants were assigned sequentially to receive two doses of either 25 μg or 100 μg of vaccine administered 28 days apart.
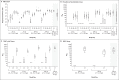
Results: Solicited adverse events were predominantly mild or moderate in severity and most frequently included fatigue, chills, headache, myalgia, and pain at the injection site. Such adverse events were dose-dependent and were more common after the second immunization. Binding-antibody responses increased rapidly after the first immunization. By day 57, among the participants who received the 25-μg dose, the anti-S-2P geometric mean titer (GMT) was 323,945 among those between the ages of 56 and 70 years and 1,128,391 among those who were 71 years of age or older; among the participants who received the 100-μg dose, the GMT in the two age subgroups was 1,183,066 and 3,638,522, respectively. After the second immunization, serum neutralizing activity was detected in all the participants by multiple methods. Binding- and neutralizing-antibody responses appeared to be similar to those previously reported among vaccine recipients between the ages of 18 and 55 years and were above the median of a panel of controls who had donated convalescent serum. The vaccine elicited a strong CD4 cytokine response involving type 1 helper T cells.
Conclusions: In this small study involving older adults, adverse events associated with the mRNA-1273 vaccine were mainly mild or moderate. The 100-μg dose induced higher binding- and neutralizing-antibody titers than the 25-μg dose, which supports the use of the 100-μg dose in a phase 3 vaccine trial. (Funded by the National Institute of Allergy and Infectious Diseases and others; mRNA-1273 Study ClinicalTrials.gov number, NCT04283461.).
Copyright © 2020 Massachusetts Medical Society.
Figures


Comment in
-
[How effective is the COVID-19 vaccine for older persons?].Tidsskr Nor Laegeforen. 2021 Nov 15;141(18). doi: 10.4045/tidsskr.21.0748. Print 2021 Dec 14. Tidsskr Nor Laegeforen. 2021. PMID: 34911279 Norwegian. No abstract available.
Similar articles
-
An mRNA Vaccine against SARS-CoV-2 - Preliminary Report.N Engl J Med. 2020 Nov 12;383(20):1920-1931. doi: 10.1056/NEJMoa2022483. Epub 2020 Jul 14. N Engl J Med. 2020. PMID: 32663912 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates.N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906. Epub 2020 Oct 14. N Engl J Med. 2020. PMID: 33053279 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2408863. doi: 10.1080/21645515.2024.2408863. Epub 2024 Oct 18. Hum Vaccin Immunother. 2024. PMID: 39422261 Free PMC article. Clinical Trial.
-
Global Perspectives on Immunization Against SARS-CoV-2 During Pregnancy and Priorities for Future Research: An International Consensus Paper From the World Association of Infectious Diseases and Immunological Disorders.Front Immunol. 2021 Dec 23;12:808064. doi: 10.3389/fimmu.2021.808064. eCollection 2021. Front Immunol. 2021. PMID: 35003137 Free PMC article. Review.
-
Myocarditis With COVID-19 mRNA Vaccines.Circulation. 2021 Aug 10;144(6):471-484. doi: 10.1161/CIRCULATIONAHA.121.056135. Epub 2021 Jul 20. Circulation. 2021. PMID: 34281357 Free PMC article. Review.
Cited by
-
Navigating the Landscape of B Cell Mediated Immunity and Antibody Monitoring in SARS-CoV-2 Vaccine Efficacy: Tools, Strategies and Clinical Trial Insights.Vaccines (Basel). 2024 Sep 24;12(10):1089. doi: 10.3390/vaccines12101089. Vaccines (Basel). 2024. PMID: 39460256 Free PMC article. Review.
-
Adverse events following COVID-19 vaccination: A comprehensive analysis of spontaneous reporting data in Ghana.PLOS Glob Public Health. 2024 Sep 27;4(9):e0003770. doi: 10.1371/journal.pgph.0003770. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39331603 Free PMC article.
-
Genomic insights into mRNA COVID-19 vaccines efficacy: Linking genetic polymorphisms to waning immunity.Hum Vaccin Immunother. 2024 Dec 31;20(1):2399382. doi: 10.1080/21645515.2024.2399382. Epub 2024 Sep 10. Hum Vaccin Immunother. 2024. PMID: 39254005 Free PMC article.
-
COVID-19: vaccination, therapeutics and a review of the science and public health.Ann Med Surg (Lond). 2024 Jul 17;86(9):5343-5353. doi: 10.1097/MS9.0000000000002374. eCollection 2024 Sep. Ann Med Surg (Lond). 2024. PMID: 39239001 Free PMC article. Review.
-
Salivary immune responses after COVID-19 vaccination.PLoS One. 2024 Sep 3;19(9):e0307936. doi: 10.1371/journal.pone.0307936. eCollection 2024. PLoS One. 2024. PMID: 39226256 Free PMC article.
References
-
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. September 9, 2020. (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...).
-
- Corbett KS, Edwards D, Leist SR, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. June 11, 2020. (https://www.biorxiv.org/content/10.1101/2020.06.11.145920v1). preprint. - PMC - PubMed
-
- Lang PO, Govind S, Bokum AT, et al. Immune senescence and vaccination in the elderly. Curr Top Med Chem 2013;13:2541-2550. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R38 AI140299/AI/NIAID NIH HHS/United States
- UM1AI148576/US/United States/United States
- HHSN272201500002C/US/United States/United States
- UM1Al148684-01S1/US/United States/United States
- UM1AI148684/US/United States/United States
- P51 OD011132/US/United States/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- HHSN272201500002C/AI/NIAID NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1AI148373/US/United States/United States
- P51 OD011132/OD/NIH HHS/United States
- AI149644/US/United States/United States
- U01 AI149644/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
