DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer
- PMID: 32982725
- PMCID: PMC7493073
- DOI: 10.3389/fphar.2020.01250
DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer
Abstract
Objective: Tumor metastasis and resistance to chemotherapy are two critical factors that contribute to the high death rate of colorectal cancer (CRC) patients. Metastasis is facilitated by the epithelial-mesenchymal transition (EMT) of tumor cells, which has emerged not only as a fundamental process during metastasis, but is also a key process leading to chemoresistance of cancer cells. However, the underlying mechanisms of EMT in CRC cell remain unknown. Here, we aim to assess the role of dual serine/threonine and tyrosine protein kinase (DSTYK) in CRC metastasis and chemoresistance.
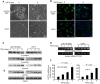
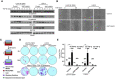
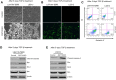
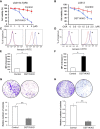
Methods: To study the role of DSTYK in TGF-β-induced EMT, we employed techniques including Crispr/Cas9 knockout (KO) to generate DSTYK KO cell lines, RT-PCR to detect the mRNA expression, immunofluorescence analyses, and western blots to detect protein levels of DSTYK in the following 4 cell lines: control LS411N-TβRII and LS411N-TβRII/DSTYK KO, control LS513 and LS513/DSTYK KO cells, treated with/without TGF-β. The effects of DSTYK on apoptosis were investigated by MTT assays, flow cytometry assays, and TUNEL assays. The expression of DSTYK in CRC patients and its correlation with EMT markers were determined by bioinformatics analysis. For in vivo analysis, both xenograft and orthotopic tumor mouse models were employed to investigate the function of DSTYK in chemoresistance and metastasis of tumors.
Results: In this study, we demonstrate that the novel kinase DSTYK promotes both TGF-β-induced EMT and the subsequent chemoresistance in CRC cells. DSTYK KO significantly attenuates TGF-β-induced EMT and chemoresistance in CRC cells. According to the Gene Expression Omnibus (GEO) database, the expression of DSTYK is not only positively correlated to the expression of TGF-β, but proportional to the death rate of CRC patients as well. Evidently, the expression of DSTYK in the metastatic colorectal cancer samples from patients was significantly higher than that of primary colorectal cancer samples. Further, we demonstrate in mouse models that chemotherapeutic drug treatment suppresses the growth of DSTYK KO tumors more effectively than control tumors.
Conclusion: Our findings identify DSTYK as a novel protein kinase in regulating TGF-β-mediated EMT and chemoresistance in CRC cells, which defines DSTYK as a potential therapeutic target for CRC therapy.
Keywords: chemoresistance; colorectal cancer; dual serine/threonine and tyrosine protein kinase; epithelial-mesenchymal transition; metastasis; transforming growth factor-β.
Copyright © 2020 Zhang, Miller, Musich, Thomas, Yao, Xie, Howe and Jiang.
Figures

Similar articles
-
DSTYK Enhances Chemoresistance in Triple-Negative Breast Cancer Cells.Cells. 2021 Dec 29;11(1):97. doi: 10.3390/cells11010097. Cells. 2021. PMID: 35011659 Free PMC article.
-
Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article.
-
S100A8 promotes epithelial-mesenchymal transition and metastasis under TGF-β/USF2 axis in colorectal cancer.Cancer Commun (Lond). 2021 Feb;41(2):154-170. doi: 10.1002/cac2.12130. Epub 2021 Jan 3. Cancer Commun (Lond). 2021. PMID: 33389821 Free PMC article.
-
Traditional Chinese Medicines as Effective Reversals of Epithelial-Mesenchymal Transition Induced-Metastasis of Colorectal Cancer: Molecular Targets and Mechanisms.Front Pharmacol. 2022 Mar 4;13:842295. doi: 10.3389/fphar.2022.842295. eCollection 2022. Front Pharmacol. 2022. PMID: 35308223 Free PMC article. Review.
-
TGF-β in correlation with tumor progression, immunosuppression and targeted therapy in colorectal cancer.Med Oncol. 2023 Oct 19;40(11):335. doi: 10.1007/s12032-023-02204-5. Med Oncol. 2023. PMID: 37855975 Review.
Cited by
-
Role of glycosyltransferases in carcinogenesis; growth factor signaling and EMT/MET programs.Glycoconj J. 2022 Apr;39(2):167-176. doi: 10.1007/s10719-022-10041-3. Epub 2022 Jan 28. Glycoconj J. 2022. PMID: 35089466 Free PMC article. Review.
-
CT45A1 promotes the metastasis of osteosarcoma cells in vitro and in vivo through β-catenin.Cell Death Dis. 2021 Jun 25;12(7):650. doi: 10.1038/s41419-021-03935-x. Cell Death Dis. 2021. PMID: 34172717 Free PMC article.
-
Role of epithelial cell-mesenchymal transition regulators in molecular typing and prognosis of colon cancer.J Gastrointest Oncol. 2023 Apr 29;14(2):744-757. doi: 10.21037/jgo-23-49. Epub 2023 Apr 17. J Gastrointest Oncol. 2023. PMID: 37201067 Free PMC article.
-
Wnt2 Contributes to the Development of Atherosclerosis.Front Cardiovasc Med. 2021 Nov 24;8:751720. doi: 10.3389/fcvm.2021.751720. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34901211 Free PMC article.
-
DSTYK inhibition increases the sensitivity of lung cancer cells to T cell-mediated cytotoxicity.J Exp Med. 2022 Dec 5;219(12):e20220726. doi: 10.1084/jem.20220726. Epub 2022 Sep 28. J Exp Med. 2022. PMID: 36169652 Free PMC article.
References
-
- de Miranda N. F., van Dinther M., van den Akker B. E., van Wezel T., ten Dijke P., Morreau H. (2015). Transforming Growth Factor beta Signaling in Colorectal Cancer Cells With Microsatellite Instability Despite Biallelic Mutations in TGFBR2. Gastroenterology 148 (7), 1427–1437 e1428. 10.1053/j.gastro.2015.02.052 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

