SUMO promotes longevity and maintains mitochondrial homeostasis during ageing in Caenorhabditis elegans
- PMID: 32968203
- PMCID: PMC7511317
- DOI: 10.1038/s41598-020-72637-9
SUMO promotes longevity and maintains mitochondrial homeostasis during ageing in Caenorhabditis elegans
Abstract
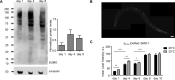
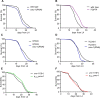
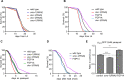
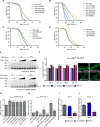
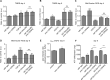
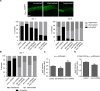
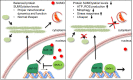
The insulin/IGF signalling pathway impacts lifespan across distant taxa, by controlling the activity of nodal transcription factors. In the nematode Caenorhabditis elegans, the transcription regulators DAF-16/FOXO and SKN-1/Nrf function to promote longevity under conditions of low insulin/IGF signalling and stress. The activity and subcellular localization of both DAF-16 and SKN-1 is further modulated by specific posttranslational modifications, such as phosphorylation and ubiquitination. Here, we show that ageing elicits a marked increase of SUMO levels in C. elegans. In turn, SUMO fine-tunes DAF-16 and SKN-1 activity in specific C. elegans somatic tissues, to enhance stress resistance. SUMOylation of DAF-16 modulates mitochondrial homeostasis by interfering with mitochondrial dynamics and mitophagy. Our findings reveal that SUMO is an important determinant of lifespan, and provide novel insight, relevant to the complexity of the signalling mechanisms that influence gene expression to govern organismal survival in metazoans.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Temporal requirements of SKN-1/NRF as a regulator of lifespan and proteostasis in Caenorhabditis elegans.PLoS One. 2021 Jul 1;16(7):e0243522. doi: 10.1371/journal.pone.0243522. eCollection 2021. PLoS One. 2021. PMID: 34197476 Free PMC article.
-
Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity.Nature. 2015 Mar 5;519(7541):97-101. doi: 10.1038/nature14021. Epub 2014 Dec 15. Nature. 2015. PMID: 25517099 Free PMC article.
-
SUMO Wrestles with Mitophagy to Extend Lifespan.Rejuvenation Res. 2020 Dec;23(6):527-532. doi: 10.1089/rej.2020.2406. Rejuvenation Res. 2020. PMID: 33256568 Review.
-
Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1.Eur J Nutr. 2020 Feb;59(1):137-150. doi: 10.1007/s00394-019-01894-w. Epub 2019 Feb 1. Eur J Nutr. 2020. PMID: 30710163
-
SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans.Free Radic Biol Med. 2015 Nov;88(Pt B):290-301. doi: 10.1016/j.freeradbiomed.2015.06.008. Epub 2015 Aug 5. Free Radic Biol Med. 2015. PMID: 26232625 Free PMC article. Review.
Cited by
-
ULP-2 SUMO protease regulates UPRmt and mitochondrial homeostasis in Caenorhabditis elegans.Free Radic Biol Med. 2024 Mar;214:19-27. doi: 10.1016/j.freeradbiomed.2024.01.050. Epub 2024 Jan 30. Free Radic Biol Med. 2024. PMID: 38301974 Free PMC article.
-
Allele-specific mitochondrial stress induced by Multiple Mitochondrial Dysfunctions Syndrome 1 pathogenic mutations modeled in Caenorhabditis elegans.PLoS Genet. 2021 Aug 27;17(8):e1009771. doi: 10.1371/journal.pgen.1009771. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34449775 Free PMC article.
-
Auto-sumoylation of the Ubc9 E2 SUMO-conjugating Enzyme Extends Cellular Lifespan.Res Sq [Preprint]. 2024 Mar 21:rs.3.rs-4016606. doi: 10.21203/rs.3.rs-4016606/v1. Res Sq. 2024. PMID: 38562857 Free PMC article. Preprint.
-
Network analysis in aged C. elegans reveals candidate regulatory genes of ageing.Biogerontology. 2021 Jun;22(3):345-367. doi: 10.1007/s10522-021-09920-3. Epub 2021 Apr 19. Biogerontology. 2021. PMID: 33871732
-
Insights Into the Links Between Proteostasis and Aging From C. elegans.Front Aging. 2022 Mar 18;3:854157. doi: 10.3389/fragi.2022.854157. eCollection 2022. Front Aging. 2022. PMID: 35821832 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

