Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19)
- PMID: 32936252
- PMCID: PMC7195692
- DOI: 10.1001/jamacardio.2020.1834
Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19)
Erratum in
-
Errors in Abstract, Results, and End Matter.JAMA Cardiol. 2020 Sep 1;5(9):1071. doi: 10.1001/jamacardio.2020.2302. JAMA Cardiol. 2020. PMID: 32936256 Free PMC article. No abstract available.
Abstract
Importance: Administration of hydroxychloroquine with or without azithromycin for the treatment of coronavirus disease 2019 (COVID-19)-associated pneumonia carries increased risk of corrected QT (QTc) prolongation and cardiac arrhythmias.
Objective: To characterize the risk and degree of QT prolongation in patients with COVID-19 in association with their use of hydroxychloroquine with or without concomitant azithromycin.
Design, setting, and participants: This was a cohort study performed at an academic tertiary care center in Boston, Massachusetts, of patients hospitalized with at least 1 positive COVID-19 nasopharyngeal polymerase chain reaction test result and clinical findings consistent with pneumonia who received at least 1 day of hydroxychloroquine from March 1, 2020, through April 7, 2020.
Main outcomes and measures: Change in QT interval after receiving hydroxychloroquine with or without azithromycin; occurrence of other potential adverse drug events.
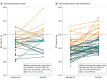
Results: Among 90 patients given hydroxychloroquine, 53 received concomitant azithromycin; 44 (48.9%) were female, and the mean (SD) body mass index was 31.5 (6.6). Hypertension (in 48 patients [53.3%]) and diabetes mellitus (in 26 patients [28.9%]) were the most common comorbid conditions. The overall median (interquartile range) baseline QTc was 455 (430-474) milliseconds (hydroxychloroquine, 473 [454-487] milliseconds vs hydroxychloroquine and azithromycin, 442 [427-461] milliseconds; P < .001). Those receiving concomitant azithromycin had a greater median (interquartile range) change in QT interval (23 [10-40] milliseconds) compared with those receiving hydroxychloroquine alone (5.5 [-15.5 to 34.25] milliseconds; P = .03). Seven patients (19%) who received hydroxychloroquine monotherapy developed prolonged QTc of 500 milliseconds or more, and 3 patients (8%) had a change in QTc of 60 milliseconds or more. Of those who received concomitant azithromycin, 11 of 53 (21%) had prolonged QTc of 500 milliseconds or more and 7 of 53 (13 %) had a change in QTc of 60 milliseconds or more. The likelihood of prolonged QTc was greater in those who received concomitant loop diuretics (adjusted odds ratio, 3.38 [95% CI, 1.03-11.08]) or had a baseline QTc of 450 milliseconds or more (adjusted odds ratio, 7.11 [95% CI, 1.75-28.87]). Ten patients had hydroxychloroquine discontinued early because of potential adverse drug events, including intractable nausea, hypoglycemia, and 1 case of torsades de pointes.
Conclusions and relevance: In this cohort study, patients who received hydroxychloroquine for the treatment of pneumonia associated with COVID-19 were at high risk of QTc prolongation, and concurrent treatment with azithromycin was associated with greater changes in QTc. Clinicians should carefully weigh risks and benefits if considering hydroxychloroquine and azithromycin, with close monitoring of QTc and concomitant medication usage.
Conflict of interest statement
Figures
Comment in
-
QT Interval in Patients With COVID-19.JAMA Cardiol. 2021 Mar 1;6(3):357-358. doi: 10.1001/jamacardio.2020.4955. JAMA Cardiol. 2021. PMID: 33084841 No abstract available.
-
QT Interval in Patients With COVID-19.JAMA Cardiol. 2021 Mar 1;6(3):357. doi: 10.1001/jamacardio.2020.4952. JAMA Cardiol. 2021. PMID: 33084843 No abstract available.
Similar articles
-
Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring.J Am Heart Assoc. 2020 Jun 16;9(12):e017144. doi: 10.1161/JAHA.120.017144. Epub 2020 May 28. J Am Heart Assoc. 2020. PMID: 32463348 Free PMC article.
-
QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin.Heart Rhythm. 2020 Sep;17(9):1425-1433. doi: 10.1016/j.hrthm.2020.05.014. Epub 2020 May 12. Heart Rhythm. 2020. PMID: 32407884 Free PMC article.
-
Cardiac Corrected QT Interval Changes Among Patients Treated for COVID-19 Infection During the Early Phase of the Pandemic.JAMA Netw Open. 2021 Apr 1;4(4):e216842. doi: 10.1001/jamanetworkopen.2021.6842. JAMA Netw Open. 2021. PMID: 33890991 Free PMC article.
-
Macrolides and viral infections: focus on azithromycin in COVID-19 pathology.Int J Antimicrob Agents. 2020 Aug;56(2):106053. doi: 10.1016/j.ijantimicag.2020.106053. Epub 2020 Jun 10. Int J Antimicrob Agents. 2020. PMID: 32534189 Free PMC article. Review.
-
Hydroxychloroquine as prophylaxis or treatment for COVID-19: What does the evidence say?Indian J Public Health. 2020 Jun;64(Supplement):S125-S127. doi: 10.4103/ijph.IJPH_496_20. Indian J Public Health. 2020. PMID: 32496241 Review.
Cited by
-
Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients.PLoS One. 2021 May 25;16(5):e0251048. doi: 10.1371/journal.pone.0251048. eCollection 2021. PLoS One. 2021. PMID: 34033655 Free PMC article.
-
Adverse drug event risk assessment by the virtual addition of COVID-19 repurposed drugs to Medicare and commercially insured patients' drug regimens: A drug safety simulation study.Clin Transl Sci. 2021 Sep;14(5):1799-1809. doi: 10.1111/cts.13025. Epub 2021 Aug 25. Clin Transl Sci. 2021. PMID: 33786990 Free PMC article.
-
Clinical Characteristics of 3 Patients Infected with COVID-19: Age, Interleukin 6 (IL-6), Lymphopenia, and Variations in Chest Computed Tomography (CT).Am J Case Rep. 2020 Oct 14;21:e924905. doi: 10.12659/AJCR.924905. Am J Case Rep. 2020. PMID: 33052896 Free PMC article.
-
Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study.Eur J Intern Med. 2020 Dec;82:38-47. doi: 10.1016/j.ejim.2020.08.019. Epub 2020 Aug 25. Eur J Intern Med. 2020. PMID: 32859477 Free PMC article.
-
Hydroxychloroquine and Coronavirus Disease 2019: A Systematic Review of a Scientific Failure.Rambam Maimonides Med J. 2020 Jul 31;11(3):e0025. doi: 10.5041/RMMJ.10416. Rambam Maimonides Med J. 2020. PMID: 32792041 Free PMC article. Review.
References
-
- US Department of Health & Human Services, Centers for Disease Control and Prevention . Coronavirus (COVID-19). Published 2020. Accessed April 10, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/index.html
-
- Multicenter Collaboration Group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for Chloroquine in the Treatment of Novel Coronavirus Pneumonia . [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E019-E9. doi:10.3760/cma.j.issn.1001-0939.2020.0019 - PubMed
-
- Molina JM, Delaugerre C, Le Goff J, et al. . No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. Published online March 30, 2020. doi:10.1016/j.medmal.2020.03.006 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

