Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration
- PMID: 32929328
- PMCID: PMC7481429
- DOI: 10.7150/thno.46639
Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration
Abstract
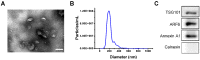
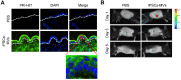
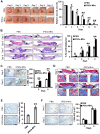
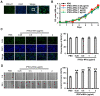
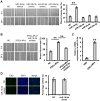
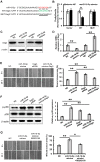
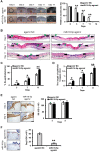
Background: Induced pluripotent stem cells (iPSCs) have emerged as a promising treatment paradigm for skin wounds. Extracellular vesicles are now recognized as key mediators of beneficial stem cells paracrine effects. In this study, we investigated the effect of iPSCs-derived microvesicles (iPSCs-MVs) on deep second-degree burn wound healing and explored the underlying mechanism. Methods: iPSCs-MVs were isolated and purified from conditioned medium of iPSCs and confirmed by electron micrograph and size distribution. In deep second-degree burn model, iPSCs-MVs were injected subcutaneously around wound sites and the efficacy was assessed by measuring wound closure areas, histological examination and immunohistochemistry staining. In vitro, CCK-8, EdU staining and scratch assays were used to assess the effects of iPSCs-MVs on proliferation and migration of keratinocytes. Next, we explored the underlying mechanisms by high-throughput microRNA sequencing. The roles of the miR-16-5p in regulation of keratinocytes function induced by iPSCs-MVs were assessed. Moreover, the target gene which mediated the biological effects of miR-16-5p in keratinocytes was also been detected. Finally, we examined the effect of local miR-16-5p treatment on deep second degree-burns wound healing in mice. Results: The local transplantation of iPSCs-MVs into the burn wound bed resulted in accelerated wound closure including the increased re-epithelialization. In vitro, iPSCs-MVs could promote the migration of keratinocytes. We also found that miR-16-5p is a critical factor in iPSCs-MVs-induced promotion of keratinocytes migration in vitro through activating p38/MARK pathway by targeting Desmoglein 3 (Dsg3). Finally, we confirmed that local miR-16-5p treatment could boost re-epithelialization during burn wound healing. Conclusion: Therefore, our results indicate that iPSCs-MVs-derived miR-16-5p may be a novel therapeutic approach for deep second-degree burn wound healing.
Keywords: burn wound healing; iPSCs; miR-16-5p; microvesicles; migration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration.J Nanobiotechnology. 2022 Jun 21;20(1):291. doi: 10.1186/s12951-022-01504-8. J Nanobiotechnology. 2022. PMID: 35729564 Free PMC article.
-
Hsp90α promotes the migration of iPSCs-derived keratinocyte to accelerate deep second-degree burn wound healing in mice.Biochem Biophys Res Commun. 2019 Nov 26;520(1):145-151. doi: 10.1016/j.bbrc.2019.09.120. Epub 2019 Sep 30. Biochem Biophys Res Commun. 2019. PMID: 31582220
-
Effect of iPSCs-derived keratinocytes on healing of full-thickness skin wounds in mice.Exp Cell Res. 2019 Dec 1;385(1):111627. doi: 10.1016/j.yexcr.2019.111627. Epub 2019 Sep 20. Exp Cell Res. 2019. PMID: 31545976
-
MiR equal than others: MicroRNA enhancement for cutaneous wound healing.J Cell Physiol. 2021 Dec;236(12):8050-8059. doi: 10.1002/jcp.30485. Epub 2021 Jun 23. J Cell Physiol. 2021. PMID: 34160067 Review.
-
The potential of human induced pluripotent stem cells for modelling diabetic wound healing in vitro.Clin Sci (Lond). 2018 Aug 14;132(15):1629-1643. doi: 10.1042/CS20171483. Print 2018 Aug 16. Clin Sci (Lond). 2018. PMID: 30108152 Review.
Cited by
-
Extracellular vesicles from 3D cultured dermal papilla cells improve wound healing via Krüppel-like factor 4/vascular endothelial growth factor A -driven angiogenesis.Burns Trauma. 2023 Oct 30;11:tkad034. doi: 10.1093/burnst/tkad034. eCollection 2023. Burns Trauma. 2023. PMID: 37908562 Free PMC article.
-
A Weakly Supervised Learning Method for Cell Detection and Tracking Using Incomplete Initial Annotations.Int J Mol Sci. 2023 Nov 7;24(22):16028. doi: 10.3390/ijms242216028. Int J Mol Sci. 2023. PMID: 38003217 Free PMC article.
-
Comprehensive Profiling of Secretome Formulations from Fetal- and Perinatal Human Amniotic Fluid Stem Cells.Int J Mol Sci. 2021 Apr 2;22(7):3713. doi: 10.3390/ijms22073713. Int J Mol Sci. 2021. PMID: 33918297 Free PMC article.
-
Identification of ferroptosis-related genes and predicted overall survival in patients with burns.Front Surg. 2023 Jan 9;9:1060036. doi: 10.3389/fsurg.2022.1060036. eCollection 2022. Front Surg. 2023. PMID: 36700031 Free PMC article.
-
Exosomal IRF1-loaded rat adipose-derived stem cell sheet contributes to wound healing in the diabetic foot ulcers.Mol Med. 2023 Apr 25;29(1):60. doi: 10.1186/s10020-023-00617-6. Mol Med. 2023. PMID: 37098476 Free PMC article.
References
-
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. - PubMed
-
- Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D. et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66:384–93. - PubMed
-
- Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M. et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

