Neuronal Signaling Involved in Neuronal Polarization and Growth: Lipid Rafts and Phosphorylation
- PMID: 32922262
- PMCID: PMC7456915
- DOI: 10.3389/fnmol.2020.00150
Neuronal Signaling Involved in Neuronal Polarization and Growth: Lipid Rafts and Phosphorylation
Abstract
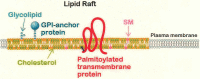
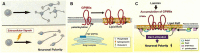
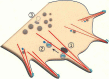
Neuronal polarization and growth are developmental processes that occur during neuronal cell differentiation. The molecular signaling mechanisms involved in these events in in vivo mammalian brain remain unclear. Also, cellular events of the neuronal polarization process within a given neuron are thought to be constituted of many independent intracellular signal transduction pathways (the "tug-of-war" model). However, in vivo results suggest that such pathways should be cooperative with one another among a given group of neurons in a region of the brain. Lipid rafts, specific membrane domains with low fluidity, are candidates for the hotspots of such intracellular signaling. Among the signals reported to be involved in polarization, a number are thought to be present or translocated to the lipid rafts in response to extracellular signals. As part of our analysis, we discuss how such novel molecular mechanisms are combined for effective regulation of neuronal polarization and growth, focusing on the significance of the lipid rafts, including results based on recently introduced methods.
Keywords: JNK; growth cone; lipid rafts; palmitoylation; phosphoproteomics; super-resolution microscopy.
Copyright © 2020 Igarashi, Honda, Kawasaki and Nozumi.
Figures

Similar articles
-
Glycoprotein M6a as a signaling transducer in neuronal lipid rafts.Neurosci Res. 2018 Mar;128:19-24. doi: 10.1016/j.neures.2017.11.002. Epub 2017 Nov 20. Neurosci Res. 2018. PMID: 29158160 Review.
-
Extracellular Signals Induce Glycoprotein M6a Clustering of Lipid Rafts and Associated Signaling Molecules.J Neurosci. 2017 Apr 12;37(15):4046-4064. doi: 10.1523/JNEUROSCI.3319-16.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275160 Free PMC article.
-
R7-binding protein targets the G protein beta 5/R7-regulator of G protein signaling complex to lipid rafts in neuronal cells and brain.BMC Biochem. 2007 Sep 19;8:18. doi: 10.1186/1471-2091-8-18. BMC Biochem. 2007. PMID: 17880698 Free PMC article.
-
The region-specific activities of lipid rafts during axon growth and guidance.J Neurochem. 2006 Jul;98(2):330-5. doi: 10.1111/j.1471-4159.2006.03888.x. J Neurochem. 2006. PMID: 16805828 Review.
-
Lipid rafts as major platforms for signaling regulation in cancer.Adv Biol Regul. 2015 Jan;57:130-46. doi: 10.1016/j.jbior.2014.10.003. Epub 2014 Oct 27. Adv Biol Regul. 2015. PMID: 25465296 Review.
Cited by
-
Advances in Understanding the Molecular Mechanisms of Neuronal Polarity.Mol Neurobiol. 2023 May;60(5):2851-2870. doi: 10.1007/s12035-023-03242-w. Epub 2023 Feb 4. Mol Neurobiol. 2023. PMID: 36738353 Review.
-
Lipid Rafts: The Maestros of Normal Brain Development.Biomolecules. 2024 Mar 18;14(3):362. doi: 10.3390/biom14030362. Biomolecules. 2024. PMID: 38540780 Free PMC article. Review.
-
One Raft to Guide Them All, and in Axon Regeneration Inhibit Them.Int J Mol Sci. 2021 May 8;22(9):5009. doi: 10.3390/ijms22095009. Int J Mol Sci. 2021. PMID: 34066896 Free PMC article. Review.
-
Molecular Mechanisms Involved in the Regulation of Neurodevelopment by miR-124.Mol Neurobiol. 2023 Jul;60(7):3569-3583. doi: 10.1007/s12035-023-03271-5. Epub 2023 Feb 25. Mol Neurobiol. 2023. PMID: 36840845 Review.
-
Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders.Antioxidants (Basel). 2021 Sep 17;10(9):1483. doi: 10.3390/antiox10091483. Antioxidants (Basel). 2021. PMID: 34573116 Free PMC article. Review.
References
-
- Aberg K. A., Dean B., Shabalin A. A., Chan R. F., Han L. K. M., Zhao M., et al. . (2020). Methylome-wide association findings for major depressive disorder overlap in blood and brain and replicate in independent brain samples. Mol. Psychiatry 25, 1344–1354. 10.1038/s41380-018-0247-6 - DOI - PMC - PubMed
-
- Assaife-Lopes N., Sousa V. C., Pereira D. B., Ribeiro J. A., Chao M. V., Sebastião A. M. (2010). Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: implications for neuromodulation. J. Neurosci. 30, 8468–8480. 10.1523/JNEUROSCI.5695-09.2010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

