Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients
- PMID: 32917154
- PMCID: PMC7488506
- DOI: 10.1186/s12885-020-07376-1
Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients
Abstract
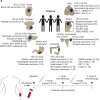
Background: Recent studies have shown that chemotherapy destabilizes the blood vasculature and increases circulating tumor cell (CTC) influx into the circulation of metastatic cancer patients (Met-pa). CTCs are a precursor of cancer metastasis, in which they can migrate as single CTCs or as CTC clusters with stromal cells such as cancer-associated fibroblasts (CAFs) as cell aggregates.
Methods: Blood samples were collected from 52 Met-pa, and the number of CTC and CAF was determined along with the temporal fluctuation of these through the chemotherapy treatment.
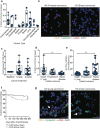
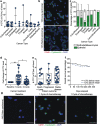
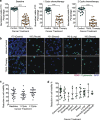
Results: In this study, CTC level was found to increase two-fold from the initial level after 1 cycle of chemotherapy and returned to baseline after 2 cycles of chemotherapy. Importantly, we determined for the first time that circulating CAF levels correlate with worse prognosis and a lower probability of survival in Met-pa. Based on the CTC release induced by chemotherapy, we evaluated the efficacy of our previously developed cancer immunotherapy to eradicate CTCs from Met-pa blood using an ex vivo approach and demonstrate this could kill over 60% of CTCs.
Conclusion: Collectively, we found that CAF levels in Met-pa serve as a predictive biomarker for cancer prognosis. Additionally, we demonstrate the efficacy of our therapy to kill primary CTCs for a range of cancer types, supporting its potential use as an anti-metastasis therapy in the clinical setting.
Keywords: Cancer prognosis; Cancer-associated fibroblast; Chemotherapy; Circulating tumor cells; TRAIL-based liposomal therapy.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures

Similar articles
-
TRAIL-coated leukocytes to kill circulating tumor cells in the flowing blood from prostate cancer patients.BMC Cancer. 2021 Aug 6;21(1):898. doi: 10.1186/s12885-021-08589-8. BMC Cancer. 2021. PMID: 34362331 Free PMC article.
-
Heterotypic clustering of circulating tumor cells and circulating cancer-associated fibroblasts facilitates breast cancer metastasis.Breast Cancer Res Treat. 2021 Aug;189(1):63-80. doi: 10.1007/s10549-021-06299-0. Epub 2021 Jul 3. Breast Cancer Res Treat. 2021. PMID: 34216317
-
Circulating tumor cells (CTCs)/circulating tumor endothelial cells (CTECs) and their subtypes in small cell lung cancer: Predictors for response and prognosis.Thorac Cancer. 2021 Oct;12(20):2749-2757. doi: 10.1111/1759-7714.14120. Epub 2021 Aug 23. Thorac Cancer. 2021. PMID: 34423906 Free PMC article.
-
Circulating tumor cells in cancer patients: developments and clinical applications for immunotherapy.Mol Cancer. 2020 Jan 24;19(1):15. doi: 10.1186/s12943-020-1141-9. Mol Cancer. 2020. PMID: 31980023 Free PMC article. Review.
-
CTC clusters in cancer progression and metastasis.Med Oncol. 2017 Jan;34(1):12. doi: 10.1007/s12032-016-0875-0. Epub 2016 Dec 23. Med Oncol. 2017. PMID: 28012133 Review.
Cited by
-
Circulating Tumour Cells (CTCs) in NSCLC: From Prognosis to Therapy Design.Pharmaceutics. 2021 Nov 5;13(11):1879. doi: 10.3390/pharmaceutics13111879. Pharmaceutics. 2021. PMID: 34834295 Free PMC article. Review.
-
The role of host response to chemotherapy: resistance, metastasis and clinical implications.Clin Exp Metastasis. 2024 Aug;41(4):495-507. doi: 10.1007/s10585-023-10243-5. Epub 2023 Nov 24. Clin Exp Metastasis. 2024. PMID: 37999904 Review.
-
Breast-to-brain metastasis is exacerbated with chemotherapy through blood-cerebrospinal fluid barrier and induces Alzheimer's-like pathology.J Neurosci Res. 2023 Dec;101(12):1900-1913. doi: 10.1002/jnr.25249. Epub 2023 Oct 3. J Neurosci Res. 2023. PMID: 37787045 Free PMC article.
-
Mobilization of Circulating Tumor Cells after Short- and Long-Term FOLFIRINOX and GEM/nab-PTX Chemotherapy in Xenograft Mouse Models of Human Pancreatic Cancer.Cancers (Basel). 2023 Nov 20;15(22):5482. doi: 10.3390/cancers15225482. Cancers (Basel). 2023. PMID: 38001741 Free PMC article.
-
Advancements in Circulating Tumor Cell Research: Bridging Biology and Clinical Applications.Cancers (Basel). 2024 Mar 20;16(6):1213. doi: 10.3390/cancers16061213. Cancers (Basel). 2024. PMID: 38539545 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

