Mechanisms of bone development and repair
- PMID: 32901139
- PMCID: PMC7699981
- DOI: 10.1038/s41580-020-00279-w
Mechanisms of bone development and repair
Abstract
Bone development occurs through a series of synchronous events that result in the formation of the body scaffold. The repair potential of bone and its surrounding microenvironment - including inflammatory, endothelial and Schwann cells - persists throughout adulthood, enabling restoration of tissue to its homeostatic functional state. The isolation of a single skeletal stem cell population through cell surface markers and the development of single-cell technologies are enabling precise elucidation of cellular activity and fate during bone repair by providing key insights into the mechanisms that maintain and regenerate bone during homeostasis and repair. Increased understanding of bone development, as well as normal and aberrant bone repair, has important therapeutic implications for the treatment of bone disease and ageing-related degeneration.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

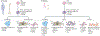

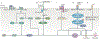
Similar articles
-
Skeletal adaptations during growth.Triangle. 1992;31(2/3):77-88. Triangle. 1992. PMID: 11539347
-
A tale of the good and bad: Cell senescence in bone homeostasis and disease.Int Rev Cell Mol Biol. 2019;346:97-128. doi: 10.1016/bs.ircmb.2019.03.005. Epub 2019 Apr 15. Int Rev Cell Mol Biol. 2019. PMID: 31122396 Review.
-
Abstracts of the 26th Annual Meeting of the American Society for Bone and Mineral Research. October 1-5, 2004, Seattle, Washington, USA.J Bone Miner Res. 2004 Oct;19 Suppl 1:S2-543. J Bone Miner Res. 2004. PMID: 15505937 No abstract available.
-
The endothelium-bone axis in development, homeostasis and bone and joint disease.Nat Rev Rheumatol. 2021 Oct;17(10):608-620. doi: 10.1038/s41584-021-00682-3. Epub 2021 Sep 3. Nat Rev Rheumatol. 2021. PMID: 34480164 Free PMC article. Review.
-
[The importance of protein growth factors for the local regulation of bone growth].Dtsch Med Wochenschr. 1990 Dec 14;115(50):1921-6. doi: 10.1055/s-2008-1065245. Dtsch Med Wochenschr. 1990. PMID: 2257782 Review. German. No abstract available.
Cited by
-
SLC38A2 provides proline and alanine to regulate postnatal bone mass accrual in mice.Front Physiol. 2022 Sep 23;13:992679. doi: 10.3389/fphys.2022.992679. eCollection 2022. Front Physiol. 2022. PMID: 36213239 Free PMC article.
-
Role of histone methyltransferase KMT2D in BMSC osteogenesis via AKT signaling.Regen Ther. 2024 Sep 10;26:775-782. doi: 10.1016/j.reth.2024.08.022. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39309396 Free PMC article.
-
Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review.Int J Mol Sci. 2023 Dec 11;24(24):17365. doi: 10.3390/ijms242417365. Int J Mol Sci. 2023. PMID: 38139194 Free PMC article. Review.
-
Challenges of Hip Arthroplasty in a Paretic, Spastic Limb: A Case Study on Managing Femoral Neck Fracture Following Fixation Failure in a Hemiparetic Patient.J Clin Med. 2024 Jul 10;13(14):4023. doi: 10.3390/jcm13144023. J Clin Med. 2024. PMID: 39064063 Free PMC article.
-
Advanced Hydrogel-Based Strategies for Enhanced Bone and Cartilage Regeneration: A Comprehensive Review.Gels. 2023 Nov 8;9(11):885. doi: 10.3390/gels9110885. Gels. 2023. PMID: 37998975 Free PMC article. Review.
References
-
- Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol 13, 27–38 (2012). - PubMed
-
- Garnero P, Sornay-Rendu E, Chapuy MC & Delmas PD Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res 11, 337–349 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

