Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants
- PMID: 32897368
- PMCID: PMC8653633
- DOI: 10.1093/cid/ciaa951
Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants
Abstract
Background: Respiratory syncytial virus (RSV) is a major cause of childhood medically attended respiratory infection (MARI).
Methods: We conducted a randomized, double-blind, placebo-controlled phase 3 trial in 1154 preterm infants of 1 or 2 doses of suptavumab, a human monoclonal antibody that can bind and block a conserved epitope on RSV A and B subtypes, for the prevention of RSV MARI. The primary endpoint was proportion of subjects with RSV-confirmed hospitalizations or outpatient lower respiratory tract infection (LRTI).
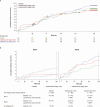
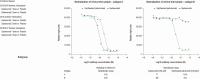
Results: There were no significant differences between primary endpoint rates (8.1%, placebo; 7.7%, 1-dose; 9.3%, 2-dose). Suptavumab prevented RSV A infections (relative risks, .38; 95% confidence interval [CI], .14-1.05 in the 1-dose group and .39 [95% CI, .14-1.07] in the 2-dose group; nominal significance of combined suptavumab group vs placebo; P = .0499), while increasing the rate of RSV B infections (relative risk 1.36 [95% CI, .73-2.56] in the 1-dose group and 1.69 [95% CI, .92-3.08] in the 2-dose group; nominal significance of combined suptavumab group vs placebo; P = .12). Sequenced RSV isolates demonstrated no suptavumab epitope changes in RSV A isolates, while all RSV B isolates had 2-amino acid substitution in the suptavumab epitope that led to loss of neutralization activity. Treatment emergent adverse events were balanced across treatment groups.
Conclusions: Suptavumab did not reduce overall RSV hospitalizations or outpatient LRTI because of a newly circulating mutant strain of RSV B. Genetic variation in circulating RSV strains will continue to challenge prevention efforts.
Clinical trials registration: NCT02325791.
Keywords: efficacy; infants; respiratory syncytial virus; safety.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
How Viral Sequence Analysis May Guide Development of Respiratory Syncytial Virus Monoclonal Antibodies.Clin Infect Dis. 2021 Dec 6;73(11):e4409-e4410. doi: 10.1093/cid/ciaa944. Clin Infect Dis. 2021. PMID: 32640025 No abstract available.
Similar articles
-
Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants.N Engl J Med. 2020 Jul 30;383(5):415-425. doi: 10.1056/NEJMoa1913556. N Engl J Med. 2020. PMID: 32726528 Clinical Trial.
-
Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial.Lancet Infect Dis. 2015 Dec;15(12):1398-408. doi: 10.1016/S1473-3099(15)00247-9. Epub 2015 Nov 4. Lancet Infect Dis. 2015. PMID: 26511956 Clinical Trial.
-
Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group.Pediatrics. 1998 Sep;102(3 Pt 1):531-7. Pediatrics. 1998. PMID: 9738173 Clinical Trial.
-
Respiratory Syncytial Virus: Targeting the G Protein Provides a New Approach for an Old Problem.J Virol. 2018 Jan 17;92(3):e01302-17. doi: 10.1128/JVI.01302-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29118126 Free PMC article. Review.
-
Respiratory syncytial virus disease: update on treatment and prevention.Expert Rev Anti Infect Ther. 2011 Jan;9(1):27-32. doi: 10.1586/eri.10.140. Expert Rev Anti Infect Ther. 2011. PMID: 21171875 Review.
Cited by
-
SARS-CoV-2-neutralising antibody BGB-DXP593 in mild-to-moderate COVID-19: a multicentre, randomised, double-blind, phase 2 trial.EClinicalMedicine. 2023 Mar;57:101832. doi: 10.1016/j.eclinm.2023.101832. Epub 2023 Feb 16. EClinicalMedicine. 2023. PMID: 36820098 Free PMC article.
-
A systematic review on global RSV genetic data: Identification of knowledge gaps.Rev Med Virol. 2022 May;32(3):e2284. doi: 10.1002/rmv.2284. Epub 2021 Sep 20. Rev Med Virol. 2022. PMID: 34543489 Free PMC article. Review.
-
Prospective mapping of viral mutations that escape antibodies used to treat COVID-19.Science. 2021 Feb 19;371(6531):850-854. doi: 10.1126/science.abf9302. Epub 2021 Jan 25. Science. 2021. PMID: 33495308 Free PMC article.
-
Passive Immunoprophylaxis against Respiratory Syncytial Virus in Children: Where Are We Now?Int J Mol Sci. 2021 Apr 2;22(7):3703. doi: 10.3390/ijms22073703. Int J Mol Sci. 2021. PMID: 33918185 Free PMC article. Review.
-
Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures.Infect Dis Ther. 2024 Aug;13(8):1725-1742. doi: 10.1007/s40121-024-01012-2. Epub 2024 Jul 6. Infect Dis Ther. 2024. PMID: 38971918 Free PMC article. Review.
References
-
- Mazur NI, Higgins D, Nunes MC, et al. ; Respiratory Syncytial Virus Network (ReSViNET) Foundation . The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. - PubMed
-
- IMpact-RSV. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. - PubMed
-
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:e620–38. - PubMed
-
- Adis Insight. Suptavumab. Available at: https://adisinsight.springer.com/drugs/800040470. Accessed 5 March 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

