Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine
- PMID: 32877576
- PMCID: PMC7494251
- DOI: 10.1056/NEJMoa2026920
Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine
Abstract
Background: NVX-CoV2373 is a recombinant severe acute respiratory syndrome coronavirus 2 (rSARS-CoV-2) nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant.
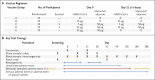
Methods: We initiated a randomized, placebo-controlled, phase 1-2 trial to evaluate the safety and immunogenicity of the rSARS-CoV-2 vaccine (in 5-μg and 25-μg doses, with or without Matrix-M1 adjuvant, and with observers unaware of trial-group assignments) in 131 healthy adults. In phase 1, vaccination comprised two intramuscular injections, 21 days apart. The primary outcomes were reactogenicity; laboratory values (serum chemistry and hematology), according to Food and Drug Administration toxicity scoring, to assess safety; and IgG anti-spike protein response (in enzyme-linked immunosorbent assay [ELISA] units). Secondary outcomes included unsolicited adverse events, wild-type virus neutralization (microneutralization assay), and T-cell responses (cytokine staining). IgG and microneutralization assay results were compared with 32 (IgG) and 29 (neutralization) convalescent serum samples from patients with Covid-19, most of whom were symptomatic. We performed a primary analysis at day 35.
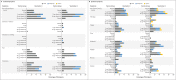
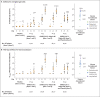
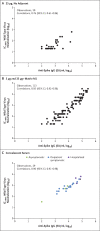
Results: After randomization, 83 participants were assigned to receive the vaccine with adjuvant and 25 without adjuvant, and 23 participants were assigned to receive placebo. No serious adverse events were noted. Reactogenicity was absent or mild in the majority of participants, more common with adjuvant, and of short duration (mean, ≤2 days). One participant had mild fever that lasted 1 day. Unsolicited adverse events were mild in most participants; there were no severe adverse events. The addition of adjuvant resulted in enhanced immune responses, was antigen dose-sparing, and induced a T helper 1 (Th1) response. The two-dose 5-μg adjuvanted regimen induced geometric mean anti-spike IgG (63,160 ELISA units) and neutralization (3906) responses that exceeded geometric mean responses in convalescent serum from mostly symptomatic Covid-19 patients (8344 and 983, respectively).
Conclusions: At 35 days, NVX-CoV2373 appeared to be safe, and it elicited immune responses that exceeded levels in Covid-19 convalescent serum. The Matrix-M1 adjuvant induced CD4+ T-cell responses that were biased toward a Th1 phenotype. (Funded by the Coalition for Epidemic Preparedness Innovations; ClinicalTrials.gov number, NCT04368988).
Copyright © 2020 Massachusetts Medical Society.
Figures
Similar articles
-
Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: A phase 2 randomized placebo-controlled trial.PLoS Med. 2021 Oct 1;18(10):e1003769. doi: 10.1371/journal.pmed.1003769. eCollection 2021 Oct. PLoS Med. 2021. PMID: 34597298 Free PMC article. Clinical Trial.
-
Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2022 Nov;22(11):1565-1576. doi: 10.1016/S1473-3099(22)00420-0. Epub 2022 Aug 10. Lancet Infect Dis. 2022. PMID: 35963274 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine in people living with and without HIV-1 infection: a randomised, controlled, phase 2A/2B trial.Lancet HIV. 2022 May;9(5):e309-e322. doi: 10.1016/S2352-3018(22)00041-8. Lancet HIV. 2022. PMID: 35489376 Free PMC article. Clinical Trial.
-
Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine.Expert Rev Vaccines. 2023 Jan-Dec;22(1):501-517. doi: 10.1080/14760584.2023.2218913. Expert Rev Vaccines. 2023. PMID: 37246757 Review.
-
Exploring the possible use of saponin adjuvants in COVID-19 vaccine.Hum Vaccin Immunother. 2020 Dec 1;16(12):2944-2953. doi: 10.1080/21645515.2020.1833579. Epub 2020 Dec 9. Hum Vaccin Immunother. 2020. PMID: 33295829 Free PMC article. Review.
Cited by
-
Review of Covid-19 vaccine clinical trials - A puzzle with missing pieces.Int J Biol Sci. 2021 Apr 10;17(6):1461-1468. doi: 10.7150/ijbs.59170. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907509 Free PMC article. Review.
-
COVID-19: vaccine's progress.Microb Biotechnol. 2021 Jul;14(4):1246-1257. doi: 10.1111/1751-7915.13818. Epub 2021 May 7. Microb Biotechnol. 2021. PMID: 33960659 Free PMC article.
-
COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations.Front Immunol. 2021 Apr 12;12:669339. doi: 10.3389/fimmu.2021.669339. eCollection 2021. Front Immunol. 2021. PMID: 33912196 Free PMC article. Review.
-
Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies.J Exp Med. 2020 Oct 5;217(10):e20200674. doi: 10.1084/jem.20200674. J Exp Med. 2020. PMID: 32910820 Free PMC article. Review.
-
Differences in the immune response elicited by two immunization schedules with an inactivated SARS-CoV-2 vaccine in a randomized phase 3 clinical trial.Elife. 2022 Oct 13;11:e81477. doi: 10.7554/eLife.81477. Elife. 2022. PMID: 36226829 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Pneumonia of unknown cause — China. 2020. (https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china).
-
- World Health Organization. Coronavirus disease (COVID-19): situation report 194: data as received by WHO from national authorities by 10:00 CEST, 1 August 2020. 2020. (https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...).
-
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
