The mTOR/ULK1 signaling pathway mediates the autophagy-promoting and osteogenic effects of dicalcium silicate nanoparticles
- PMID: 32867795
- PMCID: PMC7457372
- DOI: 10.1186/s12951-020-00663-w
The mTOR/ULK1 signaling pathway mediates the autophagy-promoting and osteogenic effects of dicalcium silicate nanoparticles
Abstract
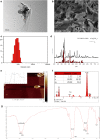
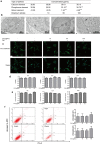
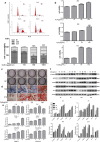
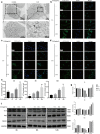
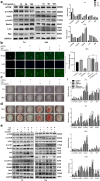
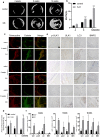
A novel bioactive inorganic material containing silicon, calcium and oxygen, calcium silicate (Ca2SiO4, C2S) with a CaO-SiO2 ingredient, has been identified as a potential candidate for artificial bone. Autophagy has an essential function in adult tissue homoeostasis and tumorigenesis. However, little is known about whether silicate nanoparticles (C2S NPs) promote osteoblastic differentiation by inducing autophagy. Here we investigated the effects of C2S NPs on bone marrow mesenchymal stem cell differentiation (BMSCs) in osteoblasts. Furthermore, we identified the osteogenic gene and protein expression in BMSCs treated with C2S NPs. We found that autophagy is important for the ability of C2S NPs to induce osteoblastic differentiation of BMSCs. Our results showed that treatment with C2S NPs upregulated the expression of BMP2, UNX2, and OSX in BMSCs, and significantly promoted the expression of LC3 and Beclin, while P62 (an autophagy substrate) was downregulated. C2S NP treatment could also enhance Alizarin red S dye (ARS), although alkaline phosphatase (ALP) activity was not significantly changed. However, all these effects could be partially reversed by 3-MA. We then detected potential signaling pathways involved in this biological effect and found that C2S NPs could activate autophagy by suppressing mTOR and facilitating ULK1 expression. Autophagy further activated β-catenin expression and promoted osteogenic differentiation. In conclusion, C2S NPs promote bone formation and osteogenic differentiation in BMSCs by activating autophagy. They achieve this effect by activating mTOR/ULK1, inducing autophagy, and subsequently triggering the WNT/β-catenin pathway to boost the differentiation and biomineralization of osteoblasts.
Keywords: Autophagy; Dicalcium silicate; MTOR/ULK1; Osteogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Dicalcium silicate microparticles modulate the differential expression of circRNAs and mRNAs in BMSCs and promote osteogenesis via circ_1983-miR-6931-Gas7 interaction.Biomater Sci. 2020 Jul 7;8(13):3664-3677. doi: 10.1039/d0bm00459f. Epub 2020 May 28. Biomater Sci. 2020. PMID: 32463418
-
Alpinetin alleviates osteoporosis by promoting osteogenic differentiation in BMSCs by triggering autophagy via PKA/mTOR/ULK1 signaling.Phytother Res. 2023 Jan;37(1):252-270. doi: 10.1002/ptr.7610. Epub 2022 Sep 14. Phytother Res. 2023. PMID: 36104214 Free PMC article.
-
Mineral trioxide aggregate upregulates odonto/osteogenic capacity of bone marrow stromal cells from craniofacial bones via JNK and ERK MAPK signalling pathways.Cell Prolif. 2014 Jun;47(3):241-8. doi: 10.1111/cpr.12099. Epub 2014 Mar 17. Cell Prolif. 2014. PMID: 24635197 Free PMC article.
-
Nanoparticles induce autophagy via mTOR pathway inhibition and reactive oxygen species generation.Nanomedicine (Lond). 2020 Jun;15(14):1419-1435. doi: 10.2217/nnm-2019-0387. Epub 2020 Jun 12. Nanomedicine (Lond). 2020. PMID: 32529946 Review.
-
Multiple functions of autophagy in vascular calcification.Cell Biosci. 2021 Aug 16;11(1):159. doi: 10.1186/s13578-021-00639-9. Cell Biosci. 2021. PMID: 34399835 Free PMC article. Review.
Cited by
-
Research advances of nanomaterials for the acceleration of fracture healing.Bioact Mater. 2023 Aug 27;31:368-394. doi: 10.1016/j.bioactmat.2023.08.016. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37663621 Free PMC article. Review.
-
The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives.Biomimetics (Basel). 2024 Apr 12;9(4):230. doi: 10.3390/biomimetics9040230. Biomimetics (Basel). 2024. PMID: 38667241 Free PMC article. Review.
-
c-MYC-induced long noncoding RNA MEG3 aggravates kidney ischemia-reperfusion injury through activating mitophagy by upregulation of RTKN to trigger the Wnt/β-catenin pathway.Cell Death Dis. 2021 Feb 18;12(2):191. doi: 10.1038/s41419-021-03466-5. Cell Death Dis. 2021. PMID: 33602903 Free PMC article.
-
The Role of Mitochondrial Homeostasis in Mesenchymal Stem Cell Therapy-Potential Implications in the Treatment of Osteogenesis Imperfecta.Pharmaceuticals (Basel). 2024 Sep 29;17(10):1297. doi: 10.3390/ph17101297. Pharmaceuticals (Basel). 2024. PMID: 39458939 Free PMC article. Review.
-
Bioactive ceramic-based materials: beneficial properties and potential applications in dental repair and regeneration.Regen Med. 2024 May 3;19(5):257-278. doi: 10.1080/17460751.2024.2343555. Epub 2024 May 22. Regen Med. 2024. PMID: 39118532 Review.
References
-
- Velasquez P, Luklinska ZB, Meseguer-Olmo L, de Val JEMS, Delgado-Ruiz RA, Calvo-Guirado JL, Ramirez-Fernandez MP, de Aza PN. TCP ceramic doped with dicalcium silicate for bone regeneration applications prepared by powder metallurgy method: in vitro and in vivo studies. J Biomed Mater Res Part A. 2013;101(7):1943–1954. doi: 10.1002/jbm.a.34495. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

