Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis
- PMID: 32867200
- PMCID: PMC7564220
- DOI: 10.3390/cancers12092438
Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis
Abstract
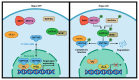
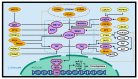
The Hippo pathway is an emerging tumor suppressor signaling pathway involved in a wide range of cellular processes. Dysregulation of different components of the Hippo signaling pathway is associated with a number of diseases including cancer. Therefore, identification of the Hippo pathway regulators and the underlying mechanism of its regulation may be useful to uncover new therapeutics for cancer therapy. The Hippo signaling pathway includes a set of kinases that phosphorylate different proteins in order to phosphorylate and inactivate its main downstream effectors, YAP and TAZ. Thus, modulating phosphorylation and dephosphorylation of the Hippo components by kinases and phosphatases play critical roles in the regulation of the signaling pathway. While information regarding kinase regulation of the Hippo pathway is abundant, the role of phosphatases in regulating this pathway is just beginning to be understood. In this review, we summarize the most recent reports on the interaction of phosphatases and the Hippo pathway in tumorigenesis. We have also introduced challenges in clarifying the role of phosphatases in the Hippo pathway and future direction of crosstalk between phosphatases and the Hippo pathway.
Keywords: Hippo pathway; LATS; MST; TAZ; YAP; protein phosphatase; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A kinome-wide screen using a NanoLuc LATS luminescent biosensor identifies ALK as a novel regulator of the Hippo pathway in tumorigenesis and immune evasion.FASEB J. 2019 Nov;33(11):12487-12499. doi: 10.1096/fj.201901343R. Epub 2019 Aug 20. FASEB J. 2019. PMID: 31431076 Free PMC article.
-
Identification of PTPN12 Phosphatase as a Novel Negative Regulator of Hippo Pathway Effectors YAP/TAZ in Breast Cancer.Int J Mol Sci. 2024 Apr 5;25(7):4064. doi: 10.3390/ijms25074064. Int J Mol Sci. 2024. PMID: 38612874 Free PMC article.
-
Complex roles of Hippo-YAP/TAZ signaling in hepatocellular carcinoma.J Cancer Res Clin Oncol. 2023 Nov;149(16):15311-15322. doi: 10.1007/s00432-023-05272-2. Epub 2023 Aug 22. J Cancer Res Clin Oncol. 2023. PMID: 37608027 Review.
-
Arhgef7 promotes activation of the Hippo pathway core kinase Lats.EMBO J. 2014 Dec 17;33(24):2997-3011. doi: 10.15252/embj.201490230. Epub 2014 Nov 25. EMBO J. 2014. PMID: 25425573 Free PMC article.
-
Non-hippo kinases: indispensable roles in YAP/TAZ signaling and implications in cancer therapy.Mol Biol Rep. 2023 May;50(5):4565-4578. doi: 10.1007/s11033-023-08329-0. Epub 2023 Mar 6. Mol Biol Rep. 2023. PMID: 36877351 Review.
Cited by
-
A Framework of Major Tumor-Promoting Signal Transduction Pathways Implicated in Melanoma-Fibroblast Dialogue.Cancers (Basel). 2020 Nov 17;12(11):3400. doi: 10.3390/cancers12113400. Cancers (Basel). 2020. PMID: 33212834 Free PMC article. Review.
-
Cuproptosis related ceRNA axis AC008083.2/miR-142-3p promotes the malignant progression of nasopharyngeal carcinoma through STRN3.PeerJ. 2024 Aug 12;12:e17859. doi: 10.7717/peerj.17859. eCollection 2024. PeerJ. 2024. PMID: 39148682 Free PMC article.
-
miR21 modulates the Hippo signaling pathway via interference with PP2A Bβ to inhibit trophoblast invasion and cause preeclampsia.Mol Ther Nucleic Acids. 2022 Sep 20;30:143-161. doi: 10.1016/j.omtn.2022.09.006. eCollection 2022 Dec 13. Mol Ther Nucleic Acids. 2022. PMID: 36250210 Free PMC article.
-
Hereditable variants of classical protein tyrosine phosphatase genes: Will they prove innocent or guilty?Front Cell Dev Biol. 2023 Jan 23;10:1051311. doi: 10.3389/fcell.2022.1051311. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36755664 Free PMC article. Review.
-
Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases.Acta Pharm Sin B. 2022 Apr;12(4):1740-1760. doi: 10.1016/j.apsb.2022.01.007. Epub 2022 Jan 19. Acta Pharm Sin B. 2022. PMID: 35847511 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

