Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients
- PMID: 32861333
- PMCID: PMC7368917
- DOI: 10.1016/j.mayocp.2020.06.028
Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients
Abstract
Objective: To provide an update on key safety metrics after transfusion of convalescent plasma in hospitalized coronavirus 2019 (COVID-19) patients, having previously demonstrated safety in 5000 hospitalized patients.
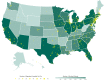
Patients and methods: From April 3 to June 2, 2020, the US Food and Drug Administration Expanded Access Program for COVID-19 convalescent plasma transfused a convenience sample of 20,000 hospitalized patients with COVID-19 convalescent plasma.
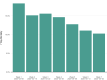
Results: The incidence of all serious adverse events was low; these included transfusion reactions (n=78; <1%), thromboembolic or thrombotic events (n=113; <1%), and cardiac events (n=677, ~3%). Notably, the vast majority of the thromboembolic or thrombotic events (n=75) and cardiac events (n=597) were judged to be unrelated to the plasma transfusion per se. The 7-day mortality rate was 13.0% (12.5%, 13.4%), and was higher among more critically ill patients relative to less ill counterparts, including patients admitted to the intensive care unit versus those not admitted (15.6 vs 9.3%), mechanically ventilated versus not ventilated (18.3% vs 9.9%), and with septic shock or multiple organ dysfunction/failure versus those without dysfunction/failure (21.7% vs 11.5%).
Conclusion: These updated data provide robust evidence that transfusion of convalescent plasma is safe in hospitalized patients with COVID-19, and support the notion that earlier administration of plasma within the clinical course of COVID-19 is more likely to reduce mortality.
Copyright © 2020. Published by Elsevier Inc.
Figures
Comment in
-
Limitations of Safety Update on Convalescent Plasma Transfusion in COVID-19 Patients.Mayo Clin Proc. 2020 Dec;95(12):2801-2802. doi: 10.1016/j.mayocp.2020.09.033. Epub 2020 Oct 1. Mayo Clin Proc. 2020. PMID: 33276848 Free PMC article. No abstract available.
-
In Reply - Limitations of Safety Update on Convalescent Plasma Transfusion in COVID-19 Patients.Mayo Clin Proc. 2020 Dec;95(12):2802-2803. doi: 10.1016/j.mayocp.2020.09.032. Epub 2020 Oct 1. Mayo Clin Proc. 2020. PMID: 33276849 Free PMC article. No abstract available.
-
In Reply - Micro-Thrombosis, Perfusion Defects, and Worsening Oxygenation in COVID-19 Patients: A Word of Caution on the Use of Convalescent Plasma.Mayo Clin Proc. 2021 Jan;96(1):259-261. doi: 10.1016/j.mayocp.2020.10.036. Epub 2020 Nov 3. Mayo Clin Proc. 2021. PMID: 33413824 Free PMC article. No abstract available.
-
Micro-Thrombosis, Perfusion Defects, and Worsening Oxygenation in COVID-19 Patients: A Word of Caution on the Use of Convalescent Plasma.Mayo Clin Proc. 2021 Jan;96(1):259. doi: 10.1016/j.mayocp.2020.10.035. Epub 2020 Nov 3. Mayo Clin Proc. 2021. PMID: 33413825 Free PMC article. No abstract available.
-
COVID-19 convalescent plasma therapy: hit fast, hit hard!Vox Sang. 2021 Oct;116(9):935-942. doi: 10.1111/vox.13091. Epub 2021 Apr 1. Vox Sang. 2021. PMID: 33794556 Free PMC article. No abstract available.
Similar articles
-
Early safety indicators of COVID-19 convalescent plasma in 5000 patients.J Clin Invest. 2020 Sep 1;130(9):4791-4797. doi: 10.1172/JCI140200. J Clin Invest. 2020. PMID: 32525844 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
-
Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks.Clin Microbiol Infect. 2020 Oct;26(10):1436-1446. doi: 10.1016/j.cmi.2020.08.005. Epub 2020 Aug 11. Clin Microbiol Infect. 2020. PMID: 32791241 Free PMC article. Review.
-
Deployment of convalescent plasma for the prevention and treatment of COVID-19.J Clin Invest. 2020 Jun 1;130(6):2757-2765. doi: 10.1172/JCI138745. J Clin Invest. 2020. PMID: 32254064 Free PMC article. Review.
Cited by
-
Distribution of age, sex, race, and ethnicity in COVID-19 clinical drug trials in the United States: A review.Contemp Clin Trials. 2022 Dec;123:106997. doi: 10.1016/j.cct.2022.106997. Epub 2022 Nov 8. Contemp Clin Trials. 2022. PMID: 36368481 Free PMC article. Review.
-
Global Perspective on COVID-19 Therapies, Cardiovascular Outcomes, and Implications for Long COVID: A State-of-the-Art Review.J Community Hosp Intern Med Perspect. 2024 Mar 4;14(2):58-66. doi: 10.55729/2000-9666.1308. eCollection 2024. J Community Hosp Intern Med Perspect. 2024. PMID: 38966504 Free PMC article. Review.
-
Convalescent Plasma for Hospitalized COVID-19 Patients: A Single-Center Experience.Life (Basel). 2022 Mar 14;12(3):420. doi: 10.3390/life12030420. Life (Basel). 2022. PMID: 35330170 Free PMC article.
-
Success rate of Remdesivir, Convalescent Plasma, and Tocilizumab in moderate to severe Covid-19 pneumonia: our experience in a tertiary care center.J Family Med Prim Care. 2021 Nov;10(11):4236-4241. doi: 10.4103/jfmpc.jfmpc_578_21. Epub 2021 Nov 29. J Family Med Prim Care. 2021. PMID: 35136795 Free PMC article.
-
A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19.Narra J. 2022 Dec;2(3):e92. doi: 10.52225/narra.v2i3.92. Epub 2022 Dec 8. Narra J. 2022. PMID: 38449903 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 4, 2020.
-
- Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. 2020. https://doi.org/10.1007/s00134-020-06047-w Intensive Care Med [Epub ahead of print] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

