Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis
- PMID: 32855863
- PMCID: PMC7422913
- DOI: 10.1167/tvst.9.8.16
Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis
Abstract
Purpose: The purpose of this study was to determine mesenchymal stem cell (MSC) therapy efficacy on rescuing the visual system in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) and to provide new mechanistic insights.
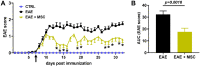
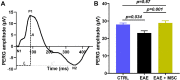
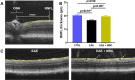
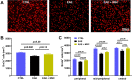
Methods: EAE was induced in female C57BL6 mice by immunization with myelin oligodendrocyte glycoprotein (MOG)35-55, complete Freund's adjuvant, and pertussis toxin. The findings were compared to sham-immunized mice. Half of the EAE mice received intraperitoneally delivered stem cells (EAE + MSC). Clinical progression was monitored according to a five-point EAE scoring scheme. Pattern electroretinogram (PERG) and retinal nerve fiber layer (RNFL) thickness were measured 32 days after induction. Retinas were harvested to determine retinal ganglion cell (RGC) density and prepared for RNA-sequencing.
Results: EAE animals that received MSC treatment seven days after EAE induction showed significantly lower motor-sensory impairment, improvement in the PERG amplitude, and preserved RNFL. Analysis of RNA-sequencing data demonstrated statistically significant differences in gene expression in the retina of MSC-treated EAE mice. Differentially expressed genes were enriched for pathways involved in endoplasmic reticulum stress, endothelial cell differentiation, HIF-1 signaling, and cholesterol transport in the MSC-treated EAE group.
Conclusions: Systemic MSC treatment positively affects RGC function and survival in EAE mice. Better cholesterol handling by increased expression of Abca1, the cholesterol efflux regulatory protein, paired with the resolution of HIF-1 signaling activation might explain the improvements seen in PERG of EAE animals after MSC treatment.
Translational relevance: Using MSC therapy in a mouse model of MS, we discovered previously unappreciated biochemical pathways associated with RGC neuroprotection, which have the potential to be pharmacologically targeted as a new treatment regimen.
Keywords: EAE; mesenchymal stem cells; optic neuritis; pattern-ERG; retinal ganglion cells.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: O.W. Gramlich, None; A.J. Brown, None; C.R. Godwin, None; M.S. Chimenti, None; L.K. Boland, None; J.A. Ankrum, None; R.H. Kardon, None
Figures
Similar articles
-
Acriflavine, a HIF-1 inhibitor, preserves vision in an experimental autoimmune encephalomyelitis model of optic neuritis.Front Immunol. 2023 Oct 23;14:1271118. doi: 10.3389/fimmu.2023.1271118. eCollection 2023. Front Immunol. 2023. PMID: 37942317 Free PMC article.
-
Correlation of Visual System Biomarkers With Motor Deficits in Experimental Autoimmune Encephalomyelitis-Optic Neuritis.Transl Vis Sci Technol. 2024 Aug 1;13(8):1. doi: 10.1167/tvst.13.8.1. Transl Vis Sci Technol. 2024. PMID: 39087931 Free PMC article.
-
Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis.Mol Vis. 2016 Dec 30;22:1503-1513. eCollection 2016. Mol Vis. 2016. PMID: 28050123 Free PMC article.
-
Monitoring retinal changes with optical coherence tomography predicts neuronal loss in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2019 Nov 4;16(1):203. doi: 10.1186/s12974-019-1583-4. J Neuroinflammation. 2019. PMID: 31684959 Free PMC article.
-
Laquinimod protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model.J Neuroinflammation. 2018 Jun 14;15(1):183. doi: 10.1186/s12974-018-1208-3. J Neuroinflammation. 2018. PMID: 29903027 Free PMC article.
Cited by
-
Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials.Stem Cell Res Ther. 2023 May 4;14(1):122. doi: 10.1186/s13287-023-03264-0. Stem Cell Res Ther. 2023. PMID: 37143147 Free PMC article. Review.
-
Acriflavine, a HIF-1 inhibitor, preserves vision in an experimental autoimmune encephalomyelitis model of optic neuritis.Front Immunol. 2023 Oct 23;14:1271118. doi: 10.3389/fimmu.2023.1271118. eCollection 2023. Front Immunol. 2023. PMID: 37942317 Free PMC article.
-
Targeting Cholesterol Homeostasis Improves Recovery in Experimental Optic Neuritis.Biomolecules. 2022 Oct 7;12(10):1437. doi: 10.3390/biom12101437. Biomolecules. 2022. PMID: 36291646 Free PMC article.
-
Neuroprotective Effects of Human Adipose-Derived Mesenchymal Stem Cells in Oxygen-Induced Retinopathy.Cell Transplant. 2023 Jan-Dec;32:9636897231213309. doi: 10.1177/09636897231213309. Cell Transplant. 2023. PMID: 38018498 Free PMC article.
-
Correlation of Visual System Biomarkers With Motor Deficits in Experimental Autoimmune Encephalomyelitis-Optic Neuritis.Transl Vis Sci Technol. 2024 Aug 1;13(8):1. doi: 10.1167/tvst.13.8.1. Transl Vis Sci Technol. 2024. PMID: 39087931 Free PMC article.
References
-
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015; 15: 545–558. - PubMed
-
- Korn T. Pathophysiology of multiple sclerosis. J Neurol. 2008; 255(suppl 6): 2–6. - PubMed
-
- Compston A, Coles, A. Multiple sclerosis. Lancet. 2008; 372: 1502–1517. - PubMed
-
- Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009; 16: 82–89. - PubMed
-
- Beck RW, et al. .. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326: 581–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

