SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study
- PMID: 32838346
- PMCID: PMC7340389
- DOI: 10.1016/S2666-5247(20)30089-6
SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study
Abstract
Background: In December, 2019, a novel zoonotic severe acute respiratory syndrome-related coronavirus emerged in China. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became pandemic within weeks and the number of human infections and severe cases is increasing. We aimed to investigate the susceptibilty of potential animal hosts and the risk of anthropozoonotic spill-over infections.
Methods: We intranasally inoculated nine fruit bats (Rousettus aegyptiacus), ferrets (Mustela putorius), pigs (Sus scrofa domesticus), and 17 chickens (Gallus gallus domesticus) with 105 TCID50 of a SARS-CoV-2 isolate per animal. Direct contact animals (n=3) were included 24 h after inoculation to test viral transmission. Animals were monitored for clinical signs and for virus shedding by nucleic acid extraction from nasal washes and rectal swabs (ferrets), oral swabs and pooled faeces samples (fruit bats), nasal and rectal swabs (pigs), or oropharyngeal and cloacal swabs (chickens) on days 2, 4, 8, 12, 16, and 21 after infection by quantitative RT-PCR (RT-qPCR). On days 4, 8, and 12, two inoculated animals (or three in the case of chickens) of each species were euthanised, and all remaining animals, including the contacts, were euthanised at day 21. All animals were subjected to autopsy and various tissues were collected for virus detection by RT-qPCR, histopathology immunohistochemistry, and in situ hybridisation. Presence of SARS-CoV-2 reactive antibodies was tested by indirect immunofluorescence assay and virus neutralisation test in samples collected before inoculation and at autopsy.
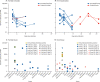
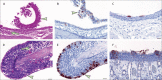
Findings: Pigs and chickens were not susceptible to SARS-CoV-2. All swabs, organ samples, and contact animals were negative for viral RNA, and none of the pigs or chickens seroconverted. Seven (78%) of nine fruit bats had a transient infection, with virus detectable by RT-qPCR, immunohistochemistry, and in situ hybridisation in the nasal cavity, associated with rhinitis. Viral RNA was also identified in the trachea, lung, and lung-associated lymphatic tissue in two animals euthanised at day 4. One of three contact bats became infected. More efficient virus replication but no clinical signs were observed in ferrets, with transmission to all three direct contact animals. Mild rhinitis was associated with viral antigen detection in the respiratory and olfactory epithelium. Prominent viral RNA loads of 0-104 viral genome copies per mL were detected in the upper respiratory tract of fruit bats and ferrets, and both species developed SARS-CoV-2-reactive antibodies reaching neutralising titres of up to 1/1024 after 21 days.
Interpretation: Pigs and chickens could not be infected intranasally by SARS-CoV-2, whereas fruit bats showed characteristics of a reservoir host. Virus replication in ferrets resembled a subclinical human infection with efficient spread. Ferrets might serve as a useful model for further studies-eg, testing vaccines or antivirals.
Funding: German Federal Ministry of Food and Agriculture.
© 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Figures
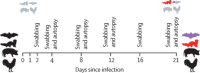
Similar articles
-
Characterisation and natural progression of SARS-CoV-2 infection in ferrets.Sci Rep. 2022 Apr 5;12(1):5680. doi: 10.1038/s41598-022-08431-6. Sci Rep. 2022. PMID: 35383204 Free PMC article.
-
SARS-CoV-2 infection and transmission via the skin to oro-nasal route with the production of bioaerosols in the ferret model.J Gen Virol. 2024 Sep;105(9):002022. doi: 10.1099/jgv.0.002022. J Gen Virol. 2024. PMID: 39292223
-
Viable SARS-CoV-2 in various specimens from COVID-19 patients.Clin Microbiol Infect. 2020 Nov;26(11):1520-1524. doi: 10.1016/j.cmi.2020.07.020. Epub 2020 Jul 23. Clin Microbiol Infect. 2020. PMID: 32711057 Free PMC article.
-
Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission.Transbound Emerg Dis. 2021 Jul;68(4):1850-1867. doi: 10.1111/tbed.13885. Epub 2020 Nov 4. Transbound Emerg Dis. 2021. PMID: 33091230 Free PMC article. Review.
-
Reverse zoonosis of coronavirus disease-19: Present status and the control by one health approach.Vet World. 2021 Oct;14(10):2817-2826. doi: 10.14202/vetworld.2021.2817-2826. Epub 2021 Oct 30. Vet World. 2021. PMID: 34903944 Free PMC article. Review.
Cited by
-
Intrinsic host susceptibility among multiple species to intranasal SARS-CoV-2 identifies diverse virological, biodistribution and pathological outcomes.Sci Rep. 2022 Nov 4;12(1):18694. doi: 10.1038/s41598-022-23339-x. Sci Rep. 2022. PMID: 36333445 Free PMC article.
-
SARS-CoV-2 Infections in Animals: Reservoirs for Reverse Zoonosis and Models for Study.Viruses. 2021 Mar 17;13(3):494. doi: 10.3390/v13030494. Viruses. 2021. PMID: 33802857 Free PMC article. Review.
-
Variation in the ACE2 receptor has limited utility for SARS-CoV-2 host prediction.Elife. 2022 Nov 23;11:e80329. doi: 10.7554/eLife.80329. Elife. 2022. PMID: 36416537 Free PMC article.
-
Susceptibility of Raccoon Dogs for Experimental SARS-CoV-2 Infection.Emerg Infect Dis. 2020 Dec;26(12):2982-2985. doi: 10.3201/eid2612.203733. Epub 2020 Oct 22. Emerg Infect Dis. 2020. PMID: 33089771 Free PMC article.
-
Monitoring SARS-CoV-2 Circulation and Diversity through Community Wastewater Sequencing, the Netherlands and Belgium.Emerg Infect Dis. 2021 May;27(5):1405-1415. doi: 10.3201/eid2705.204410. Emerg Infect Dis. 2021. PMID: 33900177 Free PMC article.
References
-
- Masters PS, Perlmen S. Coronaviridae. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 825–858.
-
- Tsang KW, Ho PL, Ooi GC. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. - PubMed
-
- Li W, Shi Z, Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

