Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19
- PMID: 32838342
- PMCID: PMC7405891
- DOI: 10.1016/j.xcrm.2020.100078
Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19
Abstract
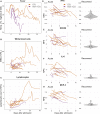
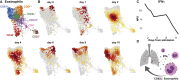
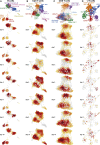
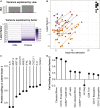
Severe disease of SARS-CoV-2 is characterized by vigorous inflammatory responses in the lung, often with a sudden onset after 5-7 days of stable disease. Efforts to modulate this hyperinflammation and the associated acute respiratory distress syndrome rely on the unraveling of the immune cell interactions and cytokines that drive such responses. Given that every patient is captured at different stages of infection, longitudinal monitoring of the immune response is critical and systems-level analyses are required to capture cellular interactions. Here, we report on a systems-level blood immunomonitoring study of 37 adult patients diagnosed with COVID-19 and followed with up to 14 blood samples from acute to recovery phases of the disease. We describe an IFNγ-eosinophil axis activated before lung hyperinflammation and changes in cell-cell co-regulation during different stages of the disease. We also map an immune trajectory during recovery that is shared among patients with severe COVID-19.
Keywords: COVID-19; SARS-CoV-2; human immunology; mass cytometry; plasma proteins; systems immunology.
© 2020 The Author(s).
Conflict of interest statement
P.B., T.L., and J.M. are the founders of Cytodelics AB, a company that commercializes reagents for blood sample preservation as used in this study.
Figures




Similar articles
-
SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity.Am J Trop Med Hyg. 2021 Jun 17;105(2):395-400. doi: 10.4269/ajtmh.20-1594. Am J Trop Med Hyg. 2021. PMID: 34143752 Free PMC article.
-
Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19.J Allergy Clin Immunol. 2021 Aug;148(2):368-380.e3. doi: 10.1016/j.jaci.2021.05.032. Epub 2021 Jun 7. J Allergy Clin Immunol. 2021. PMID: 34111453 Free PMC article.
-
Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection.Int J Infect Dis. 2021 Jun;107:221-227. doi: 10.1016/j.ijid.2021.04.080. Epub 2021 Apr 28. Int J Infect Dis. 2021. PMID: 33932604 Free PMC article.
-
Basophils and Mast Cells in COVID-19 Pathogenesis.Cells. 2021 Oct 14;10(10):2754. doi: 10.3390/cells10102754. Cells. 2021. PMID: 34685733 Free PMC article. Review.
-
Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity.Cells. 2021 Oct 29;10(11):2949. doi: 10.3390/cells10112949. Cells. 2021. PMID: 34831172 Free PMC article. Review.
Cited by
-
Molnupiravir as an Early Treatment for COVID-19: A Real Life Study.Pathogens. 2022 Sep 29;11(10):1121. doi: 10.3390/pathogens11101121. Pathogens. 2022. PMID: 36297178 Free PMC article.
-
Predictive role of blood eosinophils in adult varicella patients.Epidemiol Infect. 2022 Jun 21;150:e127. doi: 10.1017/S095026882200111X. Epidemiol Infect. 2022. PMID: 35726529 Free PMC article.
-
Guidelines for standardizing T-cell cytometry assays to link biomarkers, mechanisms, and disease outcomes in type 1 diabetes.Eur J Immunol. 2022 Mar;52(3):372-388. doi: 10.1002/eji.202049067. Epub 2022 Jan 28. Eur J Immunol. 2022. PMID: 35025103 Free PMC article.
-
High-dimensional profiling reveals phenotypic heterogeneity and disease-specific alterations of granulocytes in COVID-19.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2109123118. doi: 10.1073/pnas.2109123118. Proc Natl Acad Sci U S A. 2021. PMID: 34548411 Free PMC article.
-
Estrogen Hormone Is an Essential Sex Factor Inhibiting Inflammation and Immune Response in COVID-19.Res Sq [Preprint]. 2021 Sep 30:rs.3.rs-936900. doi: 10.21203/rs.3.rs-936900/v1. Res Sq. 2021. Update in: Sci Rep. 2022 Jun 8;12(1):9462. doi: 10.1038/s41598-022-13585-4. PMID: 34611658 Free PMC article. Updated. Preprint.
References
-
- Medzhitov R., Janeway C.A., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

